 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard ( EPA) | |
| Chemical and physical data | |
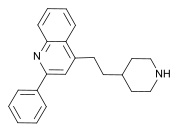
| Formula | C22H24N2 |
| Molar mass | 316.448 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
| | |
Pipequaline ( INN) (developmental code name PK-8165) is an anxiolytic drug that was never marketed. [1] It possesses a novel chemical structure that is not closely related to other drugs of this type. The drug has a similar pharmacological profile to the benzodiazepine family of drugs, but with mainly anxiolytic properties and very little sedative, amnestic or anticonvulsant effects, and so is classified as a nonbenzodiazepine anxiolytic. [2] [3] [4]
Pipequaline acts as a non-selective GABAA receptor partial agonist. [5] [6] [7] While its profile of anxiolytic effects without sedation would appear to have potential medical applications, pipequaline has never been developed for medical use and is currently only used in scientific research.
See also
References
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 986–. ISBN 978-1-4757-2085-3.
- ^ von Frenckell R, Ansseau M, Bonnet D (January 1986). "Evaluation of the sedative properties of PK 8165 (pipequaline), a benzodiazepine partial agonist, in normal subjects". International Clinical Psychopharmacology. 1 (1): 24–35. doi: 10.1097/00004850-198601000-00004. hdl: 2268/259698. PMID 3559150.
- ^ Willer JC, Von Frenkell R, Bonnet D, Le Fur G (March 1986). "The ability of PK 8165, a quinoline derivative, to reduce responses to a stressful situation in a double-blind study in man". Neuropharmacology. 25 (3): 275–81. doi: 10.1016/0028-3908(86)90252-2. PMID 3703176. S2CID 42473917.
- ^ Eves FF, Curran HV, Shine P, Lader MH (1988). "The effects on memory of pipequaline, alone or in combination with diazepam". Psychopharmacology. 95 (3): 386–9. doi: 10.1007/BF00181953. PMID 3137626. S2CID 20460757.
- ^ Mizoule J, Rataud J, Uzan A, Mazadier M, Daniel M, Gauthier A, et al. (October 1984). "Pharmacological evidence that PK 8165 behaves as a partial agonist of brain type benzodiazepine receptors". Archives Internationales de Pharmacodynamie et de Therapie. 271 (2): 189–97. PMID 6095778.
- ^ Benavides J, Malgouris C, Flamier A, Tur C, Quarteronet D, Begassat F, et al. (October 1984). "Biochemical evidence that 2-phenyl-4[(4-piperidinyl) ethyl]quinoline, a quinoline derivative with pure anticonflict properties, is a partial agonist of benzodiazepine receptors". Neuropharmacology. 23 (10): 1129–36. doi: 10.1016/0028-3908(84)90229-6. PMID 6097832. S2CID 36082385.
- ^ Debonnel G, de Montigny C (September 1987). "Pipequaline acts as a partial agonist of benzodiazepine receptors: an electrophysiological study in the hippocampus of the rat". Neuropharmacology. 26 (9): 1337–42. doi: 10.1016/0028-3908(87)90096-7. PMID 2823163. S2CID 38530030.