| |
| Names | |
|---|---|
|
IUPAC name
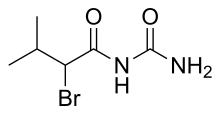
(RS)-2-Bromo-N-carbamoyl-3-methylbutanamide[
citation needed]
| |
| Identifiers | |
3D model (
JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.115 |
| EC Number |
|
| KEGG | |
| MeSH | Bromisovalum |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C6H11BrN2O2 | |
| Molar mass | 223.070 g·mol−1 |
| log P | 1.057 |
| Acidity (pKa) | 10.536 |
| Basicity (pKb) | 3.461 |
| Pharmacology | |
| N05CM03 ( WHO) | |
| Oral | |
| Related compounds | |
Related ureas
|
Carbromal |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Bromisoval ( INN), commonly known as bromovalerylurea, is a hypnotic and sedative of the bromoureide group discovered by Knoll in 1907 and patented in 1909. [1] It is marketed over the counter in Asia under various trade names (such as Brovarin [2]), usually in combination with nonsteroidal anti-inflammatory drugs.
Chronic use of bromisoval has been associated with bromine poisoning. [3] [4] [5] [6]
Bromisoval can be prepared by bromination of isovaleric acid by the Hell-Volhard-Zelinsky reaction followed by reaction with urea.
See also
References
- ^ US patent 914518, Saam, E., "Alpha-halogen-isovaleryl-urea and process of making the same", issued 1909-03-09, assigned to Knoll
- ^ "Bromisoval". International. Drugs.com.
- ^ Hashida, H.; Honda, T.; Morimoto, H.; Aibara, Y. (2001). "市販鎮痛剤常用量の服用による慢性ブロム中毒の1例" [A case of chronic bromvalerylurea intoxication due to habitual use of commercially available nonsteroidal anti-inflammatory drugs presenting an indefinite hyperchloremia] (pdf). Nihon Ronen Igakkai Zasshi. Japanese Journal of Geriatrics (in Japanese). 38 (5): 700–703. doi: 10.3143/geriatrics.38.700. ISSN 0300-9173. PMID 11605223.
- ^ Kawakami, T.; Takiyama, Y.; Yanaka, I.; Taguchi, T.; Tanaka, Y.; Nishizawa, M.; Nakano, I. (1998). "Chronic bromvalerylurea intoxication: Dystonic posture and cerebellar ataxia due to nonsteroidal anti-inflammatory drug abuse" (pdf). Internal Medicine. 37 (9). Tokyo, Japan: 788–791. doi: 10.2169/internalmedicine.37.788. PMID 9804091.
- ^ Wang, Y. -T.; Yang, S. Y.; Wu, V. C.; Wu, K. D.; Fang, C. C. (2005). "Pseudohyperchloraemia due to bromvalerylurea abuse". Nephrology Dialysis Transplantation. 20 (8): 1767–1768. doi: 10.1093/ndt/gfh945. PMID 15972320.
- ^ Arai, A.; Sato, M.; Hozumi, I.; Matsubara, N.; Tanaka, K.; Soma, Y.; Adachi, T.; Tsuji, S. (1997). "Cerebellar Ataxia and Peripheral Neuropathy due to Chronic Bromvalerylurea Poisoning" (pdf). Internal Medicine. 36 (10). Tokyo, Japan: 742–746. doi: 10.2169/internalmedicine.36.742. PMID 9372340.