| |
| Names | |
|---|---|
| Other names
dipotassium hexafluorozirconate, potassium zirconium hexafluoride, potassium fluorozirconate
| |
| Identifiers | |
3D model (
JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.037.244 |
| EC Number |
|
PubChem
CID
|
|
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| F6K2Zr | |
| Molar mass | 283.411 g·mol−1 |
| Appearance | white crystaline powder |
| Density | 3.48 g/cm3 |
| soluble | |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H301, H315, H319, H335 | |
| P301, P302, P305, P310, P330, P351, P352 | |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Potassium hexafluorozirconate is an inorganic compound of potassium, fluorine, and zirconium with the chemical formula K2ZrF6. [1] [2] [3]
Preparation
Potassium hexafluorozirconate can be prepared from precipitation from solutions:
- 2KF + ZrF4 → K2ZrF6↓
- 2KCl + (NH4)2ZrF6 → K2ZrF6↓ + 2NH4Cl
Also, in industry, it is obtained by sintering zirconium ore concentrates with K2SiF6 at 600–700 °C. [4]
Physical properties
Potassium hexafluorozirconate forms an odorless white crystalline powder.
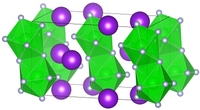
It crystallizes in the monoclinic space group C 2/c (space group No. 15). [5]
Uses
Potassium hexafluorozirconate is used as an intermediate product in the electrolytic production of metallic zirconium.
It is also used as a flame retardant for wool, [6] grain refining agent in magnesium and aluminum alloys, welding flux, and optical glass component. [7]
References
- ^ "Potassium Hexafluorozirconate". American Elements. Retrieved 26 February 2024.
- ^ "Potassium hexafluorozirconate". Sigma Aldrich. Retrieved 26 February 2024.
- ^ "Potassium Hexafluorozirconate Powder (CAS 16923-95-8)". Stanford Advanced Materials. Retrieved 26 February 2024.
- ^ Lewis, Alison; Olsen, Christine (2007). BIWIC 2007: 14th International Workshop on Industrial Crystallization : September 9th-11th, 2007, University of Cape Town, Cape Town, South Africa. IOS Press. p. 186. ISBN 978-1-58603-790-1. Retrieved 26 February 2024.
- ^ Hoppe, R.; Mehlhorn, B. (September 1976). "Die Kristallstruktur von K 2 ZrF 6". Zeitschrift für anorganische und allgemeine Chemie. 425 (3): 200–208. doi: 10.1002/zaac.19764250303. Retrieved 26 February 2024.
- ^ Lewis, David M.; Rippon, John A. (20 May 2013). The Coloration of Wool and Other Keratin Fibres. John Wiley & Sons. p. 115. ISBN 978-1-118-62509-5. Retrieved 26 February 2024.
- ^ "Potassium hexafluorozirconate | CAS 16923-95-8 | Connect Chemicals". connectchemicals.com. Retrieved 26 February 2024.