This article needs additional citations for
verification. (March 2020) |
| |
| Names | |
|---|---|
|
IUPAC name
Potassium selenide
| |
| Other names
Dipotassium selenide
| |
| Identifiers | |
3D model (
JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.013.817 |
| EC Number |
|
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| K2Se | |
| Molar mass | 157.16 |
| Appearance | clearish wet crystal [1] |
| Density | 2.29 g/cm3 [2] |
| reacts | |
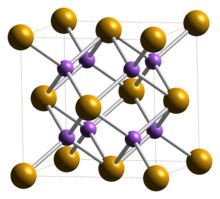
| Structure | |
| cubic: antifluorite | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
toxic |
| GHS labelling: | |



| |
| Danger | |
| H301, H331, H373, H410 | |
| P260, P262, P264, P270, P271, P273, P280, P284, P301+P310, P304+P340, P310, P314, P320, P321, P330, P361, P363, P391, P403+P233, P405, P501 | |
| Related compounds | |
Other
anions
|
Potassium oxide Potassium sulfide Potassium telluride Potassium polonide |
Other
cations
|
Lithium selenide Sodium selenide Rubidium selenide Caesium selenide |
Related compounds
|
Potassium selenate |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Potassium selenide (K2Se) is an inorganic compound formed from selenium and potassium.
Production
It can be produced by the reaction of selenium and potassium. If the two are combined in liquid ammonia, the purity is higher.
Crystal structure
Potassium selenide has a cubic, antifluorite crystal structure.
References
- ^ Jean D'Ans, Ellen Lax: Taschenbuch für Chemiker und Physiker. 3. Elemente, anorganische Verbindungen und Materialien, Minerale, Band 3. 4. Auflage, Springer, 1997, ISBN 978-3-5406-0035-0, S. 692 ( [1], p. 692, at Google Books).
- ^ Dale L. Perry, Sidney L. Phillips: Handbook of inorganic compounds. CRC Press, 1995, ISBN 978-0-8493-8671-8, S. 336 ( [2], p. 336, at Google Books).