| |
| Names | |
|---|---|
|
IUPAC names
Molybdenum(III) iodide
Molybdenum triiodide | |
| Identifiers | |
3D model (
JSmol)
|
|
| ChemSpider | |
PubChem
CID
|
|
| |
| |
| Properties | |
| MoI3 | |
| Molar mass | 476.65 g/mol |
| Appearance | black solid [1] |
| Melting point | 927 °C (1,701 °F; 1,200 K) [1] (decomposes) |
| insoluble | |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Molybdenum(III) iodide is the inorganic compound with the formula MoI3.
Preparation
Molybdenum(III) iodide is created by the reaction of molybdenum hexacarbonyl with iodine gas at 105 °C (221 °F). [2]
- 2 Mo(CO)6 + 3 I2 → 2 MoI3 + 12 CO
It can also be made from molybdenum(V) chloride and a solution of hydrogen iodide in carbon disulfide.
- MoCl5 + 5 HI → MoI3 + 5 HCl + I2
A further method is direct reaction between molybdenum metal and excess iodine at 300 °C (572 °F).
- 2 Mo + 3 I2 → 2 MoI3
As molybdenum(III) iodide is the highest stable iodide of molybdenum, this is the preferred route. [1]
Properties
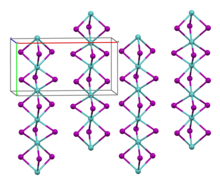
Molybdenum(III) iodide is a black antiferromagnetic solid that is air-stable at room temperature. In vacuum, it decomposes above 100 °C to molybdenum(II) iodide and iodine. It is insoluble in polar and non-polar solvents. [2] Its crystal structure is isotypic with zirconium(III) iodide. [3]
References
- ^ a b c Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 1019–1021. ISBN 978-0-08-037941-8.
- ^ a b hrsg. von Georg Brauer. Unter Mitarb. von M. Baudler (1981). Handbuch der präparativen anorganischen Chemie / 3 (3rd ed.). Stuttgart: Enke. p. 1539. ISBN 3-432-87823-0. OCLC 310719495.
- ^ Riedel, Erwin; Christoph, Janiak; Meyer, Hans-Jürgen (2012). Riedel moderne anorganische Chemie. Riedel, Erwin, 1930-, Janiak, Christoph., Meyer, Hans-Jürgen. (4. Aufl ed.). Berlin: De Gruyter. p. 357. ISBN 978-3-11-024900-2. OCLC 781540844.