From Wikipedia, the free encyclopedia
Chemical compound
HIOC
ATC code
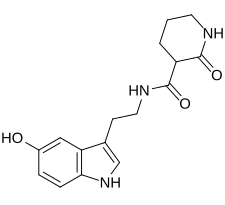
N -[2-(5-Hydroxy-1H -indol-3-yl)ethyl]-2-oxo-3-piperidinecarboxamide
CAS Number
PubChem
CID
ChemSpider
UNII
Formula C 16 H 19 N 3 O 3
Molar mass −1 3D model (
JSmol )
c1cc2c(cc1O)c(c[nH]2)CCNC(=O)C3CCCNC3=O
InChI=1S/C16H19N3O3/c20-11-3-4-14-13(8-11)10(9-19-14)5-7-18-16(22)12-2-1-6-17-15(12)21/h3-4,8-9,12,19-20H,1-2,5-7H2,(H,17,21)(H,18,22)
Key:ZIMKJLALTRLXJO-UHFFFAOYSA-N
HIOC is a
small-molecule
agent which acts as a
selective
TrkB receptor
agonist (active at at least 100 nM; prominent activation at 500 nM).
[1]
[2]
[3] It was derived from
N-acetylserotonin (NAS).
[2]
[3]
[4] Relative to NAS, HIOC possesses greater potency and a longer
half-life (~30 min or less for NAS in rats, while HIOC is still detectable up to 24 hours after administration to mice; ~4 hour half-life for HIOC in mouse brain tissues).
[2]
[3] It is described as producing long-lasting activation of the TrkB receptor and downstream
signaling kinases associated with the receptor.
[2] HIOC is
systemically active and is able to penetrate the
blood-brain-barrier .
[2] In
animal studies , HIOC was found to robustly protect against
glutamate -induced
excitotoxicity , an action which was TrkB-dependent.
[3]
A
chemical synthesis of HIOC was published in 2015.
[5]
See also References
^ Longo FM, Massa SM (July 2013). "Small-molecule modulation of neurotrophin receptors: a strategy for the treatment of neurological disease". Nature Reviews. Drug Discovery . 12 (7): 507–525.
doi :
10.1038/nrd4024 .
PMID
23977697 .
S2CID
33597483 . ^
a b c d e Iuvone PM, Boatright JH, Tosini G, Ye K (2014). "N-Acetylserotonin: Circadian Activation of the BDNF Receptor and Neuroprotection in the Retina and Brain". Retinal Degenerative Diseases . Advances in Experimental Medicine and Biology. Vol. 801. pp. 765–771.
doi :
10.1007/978-1-4614-3209-8_96 .
ISBN
978-1-4614-3208-1 PMC
4069859 .
PMID
24664769 . ^
a b c d Shen J, Ghai K, Sompol P, Liu X, Cao X, Iuvone PM, Ye K (February 2012).
"N-acetyl serotonin derivatives as potent neuroprotectants for retinas" . Proceedings of the National Academy of Sciences of the United States of America . 109 (9): 3540–3545.
Bibcode :
2012PNAS..109.3540S .
doi :
10.1073/pnas.1119201109 .
PMC
3295250 .
PMID
22331903 .
^ Tosini G, Ye K, Iuvone PM (December 2012).
"N-acetylserotonin: neuroprotection, neurogenesis, and the sleepy brain" . The Neuroscientist . 18 (6): 645–653.
doi :
10.1177/1073858412446634 .
PMC
3422380 .
PMID
22585341 .
^ Setterholm NA, McDonald FE, Boatright JH, Iuvone PM (June 2015).
"Gram-scale, chemoselective synthesis of N -[2-(5-hydroxy-1H-indol-3-yl)ethyl]-2-oxopiperidine-3-carboxamide (HIOC)" . Tetrahedron Letters . 56 (23): 3413–3415.
doi :
10.1016/j.tetlet.2015.01.167 .
PMC
4445863 .
PMID
26028783 .
Angiopoietin
CNTF
EGF (ErbB)
FGF
FGFR1
FGFR2
Agonists:
Ersofermin
FGF (
1 ,
2 (bFGF) ,
3 ,
4 ,
5 ,
6 ,
7 (
KGF ),
8 ,
9 ,
10 (KGF2) ,
17 ,
18 ,
22 )
Palifermin
Repifermin
Selpercatinib
Sprifermin
Trafermin
FGFR3
FGFR4 Unsorted
HGF (c-Met)
IGF
LNGF (p75NTR )
PDGF
RET (GFL)
SCF (c-Kit)
TGFβ
Trk
TrkA
Negative allosteric modulators:
VM-902A
TrkB
Agonists:
3,7-DHF
3,7,8,2'-THF
4'-DMA-7,8-DHF
7,3'-DHF
7,8-DHF
7,8,2'-THF
7,8,3'-THF
Amitriptyline
BDNF
BNN-20
Deoxygedunin
Deprenyl
Diosmetin
DMAQ-B1
HIOC
LM22A-4
N-Acetylserotonin
NT-3
NT-4
Norwogonin (5,7,8-THF)
R7
R13
TDP6
TrkC
VEGF Others
Additional growth factors:
Adrenomedullin
Colony-stimulating factors (see
here instead)
Connective tissue growth factor (CTGF)
Ephrins (
A1 ,
A2 ,
A3 ,
A4 ,
A5 ,
B1 ,
B2 ,
B3 )
Erythropoietin (see
here instead)
Glucose-6-phosphate isomerase (GPI; PGI, PHI, AMF)
Glia maturation factor (GMF)
Hepatoma-derived growth factor (HDGF)
Interleukins /
T-cell growth factors (see
here instead)
Leukemia inhibitory factor (LIF)
Macrophage-stimulating protein (MSP; HLP, HGFLP)
Midkine (NEGF2)
Migration-stimulating factor (MSF; PRG4)
Oncomodulin
Pituitary adenylate cyclase-activating peptide (PACAP)
Pleiotrophin
Renalase
Thrombopoietin (see
here instead)
Wnt signaling proteins Additional growth factor receptor modulators:
Cerebrolysin (neurotrophin mixture)
1-Methylpsilocin
2,alpha-DMT
2-Me-DET
2-Methyl-5-HT
2,N,N-TMT
4,5-DHP-DMT
4,5-MDO-DMT
4,5-MDO-DiPT
4-AcO-DALT
4-AcO-DET
4-AcO-DMT
4-AcO-DiPT
4-AcO-EPT
4-AcO-NMT
4-AcO-MALT
4-AcO-MET
4-AcO-DPT
4-AcO-MiPT
4-F-5-MeO-DMT
4-HO-5-MeO-DMT
4-HO-DALT
4-HO-DBT
4-HO-DET
4-HO-DiPT
4-HO-DPT
4-HO-DSBT
4-HO-EPT
4-HO-MALT
4-HO-MET
4-HO-McPT
4-HO-McPeT
4-HO-MiPT
4-HO-MPMI
4-HO-MPT
4-HO-MsBT
4-HO-NMT
4-HO-PiPT
4-HO-pyr-T
4-HO-αMT
4-Me-αET
4-Me-αMT
4-MeO-DiPT
4-MeO-DMT
4-MeO-MiPT
4-PrO-DMT
5,6-MeO-MiPT
5,6-MDO-DiPT
5,6-MDO-DMT
5,6-MDO-MiPT
5,7-Dihydroxytryptamine
5-BT
5-Bromo-DMT
5-CT
5-Chloro-αMT
5-Chloro-DMT
5-Ethoxy-αMT
5-Ethoxy-DMT
5-Ethyl-DMT
5-Fluoro-AET
5-Fluoro-αMT
5-Fluoro-DET
5-Fluoro-DMT
5-Fluoro-EPT
5-Fluoro-MET
5-HO-αMT
5-HO-DiPT
5-HTP
5-iPrO-AMT
5-MeS-DMT
5-Methoxytryptamine
5-MeO-7,N,N-TMT
5-Methyl-αET
5-MeO-2-TMT
5-MeO-αET
5-MeO-αMT
5-MeO-DALT
5-MeO-DBT
5-MeO-DET
5-MeO-DiPT
5-MeO-DMT
5-MeO-DPT
5-MeO-EiPT
5-MeO-EPT
5-MeO-MALT
5-MeO-MET
5-MeO-MiPT
5-MeO-MPMI
5-MeO-NMT
5-MeO-pyr-T
5-MeO-NBpBrT
5-Methyl-DMT
5-(Nonyloxy)tryptamine
6-Fluoro-αMT
6-Fluoro-DMT
6-Hydroxymelatonin
6-MeO-THH
7-Chloro-AMT
7-Methyl-α-ethyltryptamine
7-Methyl-DMT
Acetryptine
Aeruginascin
αET
Alpha,N-DMT
α,N,N-Trimethyltryptamine
Alpha,N,O-TMS
AL-37350A
αMT
Baeocystin
BNC-210
Bufotenidine
Bufotenin (5-HO-DMT)
BW-723C86
Convolutindole A
CP-132,484
DALT
DBT
Desformylflustrabromine
DET
DiPT
DPT
E-6801
E-6837
Ethocybin
EiPT
EMDT
EPT
FGIN-127
FGIN-143
Harmaline
HIOC
Ibogaine
Idalopirdine
Indorenate
Iprocin
Lespedamine
Luzindole
MET
Methylbutyltryptamine
MiPT
MPT
Miprocin
Melatonin
MPMI
MS-245
NAS
N-Ethyltryptamine
N-Feruloylserotonin
NMT
DMT
Norbaeocystin
Normelatonin
N-t-Butyltryptamine
O-4310
Oxypertine
Plakohypaphorine
PiPT
Psilocin (4-HO-DMT)
Psilocybin (4-PO-DMT)
Pyr-T
Rizatriptan
RU-28306
Serotonin
ST-1936
Sumatriptan
Tryptamine
Tryptophan
Yohimbine
Yuremamine
Zolmitriptan