From Wikipedia, the free encyclopedia
Chemical compound
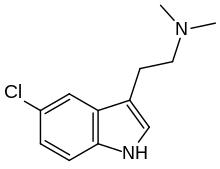
5-Chloro-N ,N -dimethyltryptamine (5-chloro-DMT ) is a
tryptamine derivative related to compounds such as
5-bromo-DMT and
5-fluoro-DMT . It acts as a
serotonin receptor
agonist and has primarily
sedative effects in animal studies.
[1]
[2]
[3] It has been sold as a
designer drug .
[4]
See also References
^ Benington F, Morin RD, Clark LC (September 1960). "Synthesis of some 5- and 6-chloro, 5-methyl, and 5,6,7-trimethyl derivatives of tryptamine". Journal of Organic Chemistry . 25 (9): 1542–1547.
doi :
10.1021/jo01079a020 .
^ Ibrahim MA, El-Alfy AT, Ezel K, Radwan MO, Shilabin AG, Kochanowska-Karamyan AJ, et al. (August 2017).
"Marine Inspired 2-(5-Halo-1H-indol-3-yl)-N,N-dimethylethanamines as Modulators of Serotonin Receptors: An Example Illustrating the Power of Bromine as Part of the Uniquely Marine Chemical Space" . Marine Drugs . 15 (8): 248.
doi :
10.3390/md15080248 .
PMC
5577603 .
PMID
28792478 .
^ Dong C, Ly C, Dunlap LE, Vargas MV, Sun J, Hwang IW, et al. (May 2021).
"Psychedelic-inspired drug discovery using an engineered biosensor" . Cell . 184 (10): 2779–2792.e18.
doi :
10.1016/j.cell.2021.03.043 .
PMC
8122087 .
PMID
33915107 .
^
"Analytical Report: 5-Cl-DMT" (PDF) . Slovenia: Nacionalni Forenzični Laboratorij. July 2020.
1-Methylpsilocin
2,alpha-DMT
2-Me-DET
2-Methyl-5-HT
2,N,N-TMT
4,5-DHP-DMT
4,5-MDO-DMT
4,5-MDO-DiPT
4-AcO-DALT
4-AcO-DET
4-AcO-DMT
4-AcO-DiPT
4-AcO-EPT
4-AcO-NMT
4-AcO-MALT
4-AcO-MET
4-AcO-DPT
4-AcO-MiPT
4-F-5-MeO-DMT
4-HO-5-MeO-DMT
4-HO-DALT
4-HO-DBT
4-HO-DET
4-HO-DiPT
4-HO-DPT
4-HO-DSBT
4-HO-EPT
4-HO-MALT
4-HO-MET
4-HO-McPT
4-HO-McPeT
4-HO-MiPT
4-HO-MPMI
4-HO-MPT
4-HO-MsBT
4-HO-NMT
4-HO-PiPT
4-HO-pyr-T
4-HO-αMT
4-Me-αET
4-Me-αMT
4-MeO-DiPT
4-MeO-DMT
4-MeO-MiPT
4-PrO-DMT
5,6-MeO-MiPT
5,6-MDO-DiPT
5,6-MDO-DMT
5,6-MDO-MiPT
5,7-Dihydroxytryptamine
5-BT
5-Bromo-DMT
5-CT
5-Chloro-αMT
5-Chloro-DMT
5-Ethoxy-αMT
5-Ethoxy-DMT
5-Ethyl-DMT
5-Fluoro-AET
5-Fluoro-αMT
5-Fluoro-DET
5-Fluoro-DMT
5-Fluoro-EPT
5-Fluoro-MET
5-HO-αMT
5-HO-DiPT
5-HTP
5-iPrO-AMT
5-MeS-DMT
5-Methoxytryptamine
5-MeO-7,N,N-TMT
5-Methyl-αET
5-MeO-2-TMT
5-MeO-αET
5-MeO-αMT
5-MeO-DALT
5-MeO-DBT
5-MeO-DET
5-MeO-DiPT
5-MeO-DMT
5-MeO-DPT
5-MeO-EiPT
5-MeO-EPT
5-MeO-MALT
5-MeO-MET
5-MeO-MiPT
5-MeO-MPMI
5-MeO-NMT
5-MeO-pyr-T
5-MeO-NBpBrT
5-Methyl-DMT
5-(Nonyloxy)tryptamine
6-Fluoro-αMT
6-Fluoro-DMT
6-Hydroxymelatonin
6-MeO-THH
7-Chloro-AMT
7-Methyl-α-ethyltryptamine
7-Methyl-DMT
Acetryptine
Aeruginascin
αET
Alpha,N-DMT
α,N,N-Trimethyltryptamine
Alpha,N,O-TMS
AL-37350A
αMT
Baeocystin
BNC-210
Bufotenidine
Bufotenin (5-HO-DMT)
BW-723C86
Convolutindole A
CP-132,484
DALT
DBT
Desformylflustrabromine
DET
DiPT
DPT
E-6801
E-6837
Ethocybin
EiPT
EMDT
EPT
FGIN-127
FGIN-143
Harmaline
HIOC
Ibogaine
Idalopirdine
Indorenate
Iprocin
Lespedamine
Luzindole
MET
Methylbutyltryptamine
MiPT
MPT
Miprocin
Melatonin
MPMI
MS-245
NAS
N-Ethyltryptamine
N-Feruloylserotonin
NMT
DMT
Norbaeocystin
Normelatonin
N-t-Butyltryptamine
O-4310
Oxypertine
Plakohypaphorine
PiPT
Psilocin (4-HO-DMT)
Psilocybin (4-PO-DMT)
Pyr-T
Rizatriptan
RU-28306
Serotonin
ST-1936
Sumatriptan
Tryptamine
Tryptophan
Yohimbine
Yuremamine
Zolmitriptan