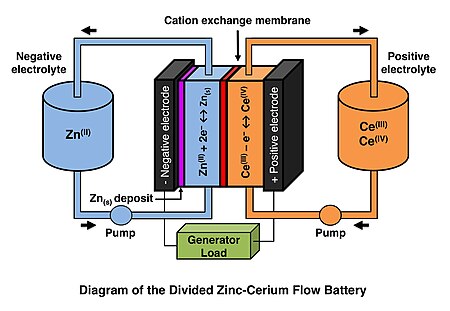
Zinc–cerium batteries are a type of redox flow battery first developed by Plurion Inc. (UK) during the 2000s. [1] [2] In this rechargeable battery, both negative zinc and positive cerium electrolytes are circulated though an electrochemical flow reactor during the operation and stored in two separated reservoirs. Negative and positive electrolyte compartments in the electrochemical reactor are separated by a cation-exchange membrane, usually Nafion ( DuPont). The Ce(III)/Ce(IV) and Zn(II)/Zn redox reactions take place at the positive and negative electrodes, respectively. Since zinc is electroplated during charge at the negative electrode this system is classified as a hybrid flow battery. Unlike in zinc–bromine and zinc–chlorine redox flow batteries, no condensation device is needed to dissolve halogen gases. The reagents used in the zinc-cerium system are considerably less expensive than those used in the vanadium flow battery.
Due to the high standard electrode potentials of both zinc and cerium redox reactions in aqueous media, the open-circuit cell voltage is as high as 2.43 V. [1] Among the other proposed rechargeable aqueous flow battery systems, this system has the largest cell voltage and its power density per electrode area is second only to H2-Br2 flow battery. [3] Methanesulfonic acid is used as supporting electrolyte, as it allows high concentrations of both zinc and cerium; the solubility of the corresponding methanesulfonates is 2.1 M for Zn, [4] 2.4 M for Ce(III) and up to 1.0 M for Ce(IV). [5] Methanesulfonic acid is particularly well suited for industrial electrochemical applications and is considered to be a green alternative to other support electrolytes. [4]
The Zn-Ce flow battery is still in early stages of development. The main technological challenge is the control of the inefficiency and self discharge (Zn corrosion via hydrogen evolution) at the negative electrode. In commercial terms, the need for expensive Pt-Ti electrodes increases the capital cost of the system in comparison to other RFBs.
Cell chemistry
At the negative electrode (anode), zinc is electroplated and stripped on the carbon polymer electrodes during charge and discharge, respectively. [6] [7] [8]
- Zn2+(aq) + 2e− ⇌ Zn(s)
- (−0.76 V vs. SHE)
At the positive electrode (cathode) (titanium based materials or carbon felt electrode), Ce(III) oxidation and Ce(IV) reduction take place during charge and discharge, respectively. [9] [10]
- Ce4+(aq) + e− ⇌ Ce3+(aq)
- (ca. +1.44 V vs. SHE)
Because of the large cell voltage, hydrogen (0 V vs. SHE) and oxygen (+1.23 V vs. SHE) could evolve theoretically as side reactions during battery operation (especially on charging). [11] The positive electrolyte is a solution of cerium(III) methanesulfonate.
History and development
The zinc–cerium redox flow battery was first proposed by Clarke and co-workers in 2004, [1] [2] which has been the core technology of Plurion Inc. (UK). In 2008, Plurion Inc. suffered a liquidity crisis and was under liquidation in 2010 and the company was formally dissolved in 2012. However, the information of the experimental conditions and charge-discharge performance described in the early patents of Plurion Inc. are limited. Since the 2010s, the electrochemical properties and the characterisation of a zinc–cerium redox flow battery have been identified by the researchers of Southampton and Strathclyde Universities. During charge/discharge cycles at 50 mA cm−2, the coulombic and voltage efficiencies of the zinc–cerium redox flow battery were reported to be 92 and 68%, respectively. [12] In 2011, a membraneless (undivided) zinc–cerium system based on low acid concentration electrolyte using compressed pieces of carbon felt positive electrode was proposed. Discharge cell voltage and energy efficiency were reported to be approximately 2.1 V and 75%, respectively. With such undivided configuration (single electrolyte compartment), self-discharge was relatively slow at low concentrations of cerium and acid. [13] [14] Major installation of the zinc–cerium redox flow battery was the > 2 kW testing facility in Glenrothes, Scotland, installed by Plurion Inc. The use of mixed acid electrolytes for the positive half-cell has been investigated as a mean to increase the kinetics of the cerium redox reaction in State Key Laboratory of Rare Earth Resource Utilization and the Jiangxi University of Science and Technology, China. [15] [16] Platinum-iridium coatings have shown the best performance as positive electrodes for the battery, while being less expensive than platinum electrodes. [17] Charge-discharge of the system has been preliminarily simulated. [18] Research on mixed acids continues and it has been shown that low concentrations of hydrochloric acid can improve the electrochemical response of the cerium reaction, while nitric acid additions had negative results. [19] Hierarchical porous carbon as the positive electrode has yielded better performance than carbon felt in laboratory scale experiments. [20] The zinc electrodeposition on the negative electrode has been studied using a Hull cell. [21] Carbon paper has also been studied as an alternative material for the positive electrode. [22] Graphene oxide-graphite composites have shown some promise as a better catalytic electrode material for the reaction of cerium in the positive electrolyte. [23] A similar cerium-lead RFB has been proposed. [24] Indium-modified electrodes have been suggested as an alternative to conventional graphitised carbon as negative electrodes. [25] The Zn-Ce system has introduced the use of this acid to other flow batteries as a better alternative to sulphuric acid. The relationship between cell potential and current density has been estimated for a Zn-Ce unit flow cell. [26] This permitted to rationalise the contribution of the thermodynamic, kinetic and ohmic components of the battery voltage and to assess the effect of increasing inter-electrode gap.
The development of the Zn-Ce battery has been reviewed, [27] as well as the electrochemical technology of cerium conversion for industrial applications, [28] which include energy storage, nuclear decontamination, indirect organic synthesis, destruction of hazardous organics and gas scrubbing.
See also
- Energy storage
- Load balancing
- Flow battery
- Rechargeable battery
- Battery (electricity)
- Electrochemical cell
- List of battery types
References
- ^ a b c R.L. Clarke, B.J. Dougherty, S. Harrison, P.J. Millington, S. Mohanta, US 2004/ 0202925 A1, Cerium Batteries, (2004).
- ^ a b R.L. Clarke, B.J. Dougherty, S. Harrison, J.P. Millington, S. Mohanta, US 2006/0063065 A1, Battery with bifunctional electrolyte, (2005).
- ^ Leung, P.K.; Ponce de León, C.; Low, C.J.T.; Walsh, F.C. (2011). "Ce(III)/Ce(IV) in methanesulfonic acid as the positive half cell of a redox flow battery". Electrochimica Acta. 56 (5): 2145–2153. doi: 10.1016/j.electacta.2010.12.038.
- ^ a b Gernon, M. D.; Wu, M.; Buszta, T.; Janney, P. (1999). "Environmental benefits of methanesulfonic acid: comparative properties and advantages". Green Chemistry. 1 (3): 127–140. doi: 10.1039/a900157c.
- ^ Kreh, R.P.; Spotnitz, R.M.; Lundquist, J.T. (1989). "Mediated electrochemical synthesis of aromatic aldehydes, ketones, and quinones using ceric methanesulfonate". The Journal of Organic Chemistry. 54 (7): 1526–1531. doi: 10.1021/jo00268a010.
- ^ Nikiforidis, G.; Berlouis, L.; Hall, D.; Hodgson, D. (2012). "Evaluation of carbon composite materials for the negative electrode in the zinc–cerium redox flow cell". Journal of Power Sources. 206: 497–503. doi: 10.1016/j.jpowsour.2011.01.036.
- ^ Nikiforidis, G.; Berlouis, L.; Hall, D.; Hodgson, D. (2013). "A study of different carbon composite materials for the negative half-cell reaction of the zinc cerium hybrid redox flow cell". Electrochimica Acta. 113: 412–423. doi: 10.1016/j.electacta.2013.09.061.
- ^ Leung, P.K.; Ponce de León, C.; Low, C.T.J.; Walsh, F.C. (2011). "Zinc deposition and dissolution in methanesulfonic acid onto a carbon composite electrode as the negative electrode reactions in a hybrid redox flow battery". Electrochimica Acta. 56 (18): 6536–6546. doi: 10.1016/j.electacta.2011.04.111.
- ^ Xie, Z.; Zhou, D.; Xiong, F.; Zhang, S.; Huang, K. (2011). "Cerium-zinc redox flow battery: Positive half-cell electrolyte studies". Journal of Rare Earths. 29 (6): 567–573. doi: 10.1016/S1002-0721(10)60499-1.
- ^ Nikiforidis, G.; Berlouis, L.; Hall, D.; Hodgson, D. (2014). "Charge/discharge cycles on Pt and Pt-Ir based electrodes for the positive side of the Zinc-Cerium hybrid redox flow battery". Electrochimica Acta. 125: 176–182. doi: 10.1016/j.electacta.2014.01.075.
- ^ Nikiforidis, G.; Berlouis, L.; Hall, D.; Hodgson, D. (2013). "Impact of electrolyte composition on the performance of the zincecerium redox flow battery system". Journal of Power Sources. 243: 691–698. doi: 10.1016/j.jpowsour.2013.06.045.
- ^ Leung, P.K.; Ponce de León, C.; Low, C.T.J.; Shah, A.A.; Walsh, F.C. (2011). "Characterization of a zinc-cerium flow battery". Journal of Power Sources. 196 (11): 5174–5185. doi: 10.1016/j.jpowsour.2011.01.095.
- ^ Leung, P.K.; Ponce-de-Leon, C.; Walsh, F.C. (2011). "An undivided zinc–cerium redox flow battery operating at room temperature (295 K)". Electrochemistry Communications. 13 (8): 770–773. doi: 10.1016/j.elecom.2011.04.011.
- ^ Leung, P.K.; Ponce de León, C.; Walsh, F.C. (2012). "The influence of operational parameters on the performance of an undivided zinc–cerium flow battery". Electrochimica Acta. 80: 7–14. doi: 10.1016/j.electacta.2012.06.074.
- ^ Xie, Z.; Xiong, F.; Zhou, D. (2011). "Study of the Ce3+/Ce4+ redox couple in mixed-acid media (CH3SO3H and H2SO4) for redox flow battery application". Energy and Fuels. 25 (5): 2399–2404. doi: 10.1021/ef200354b.
- ^ Xie, Z.; Liu, Q.; Chang, Z.; Zhang, X. (2013). "The developments and challenges of cerium half-cell in zinc–cerium redox flow battery for energy storage". Electrochimica Acta. 90: 695–704. doi: 10.1016/j.electacta.2012.12.066.
- ^ Nikiforidis, G.; Berlouis, L.; Hall, D.; Hodgson, D. (2014). "An electrochemical study on the positive electrode side of the zinc–cerium hybrid redox flow battery". Electrochimica Acta. 115: 621–629. doi: 10.1016/j.electacta.2013.09.081.
- ^ Halls, J.E.; Hawthornthwaite, A.; Hepworth, R.J.; Roberts, N.A.; Wright, K.J.; Zhou, Y.; Haswell, S.J.; Haywood, S.K.; Kelly, S.M.; Lawrence, N.S.; Wadhawan, J.D. (2013). "Empowering the smart grid: can redox batteries be matched to renewable energy systems for energy storage?" (PDF). Energy & Environmental Science. 6 (3): 1026. doi: 10.1039/c3ee23708g. hdl: 10536/DRO/DU:30063527.
- ^ Nikiforidis, G.; Daoud, W.A. (2014). "Effect of mixed acid media on the positive side of the hybrid zinc-cerium redox flow battery". Electrochimica Acta. 141: 255–262. doi: 10.1016/j.electacta.2014.06.142.
- ^ Xie, Z.; Yang, B.; Cai, D.; Yang, L. (2014). "Hierarchical porous carbon toward effective cathode in advanced zinc-cerium redox flow battery". Journal of Rare Earths. 32 (10): 973–978. doi: 10.1016/S1002-0721(14)60171-X.
- ^ Nikiforidis, G.; Cartwright, R.; Hodgson, D.; Hall, D.; Berlouis, L. (2014). "Factors affecting the performance of the Zn-Ce redox flow battery" (PDF). Electrochimica Acta. 140: 139–144. doi: 10.1016/j.electacta.2014.04.150.
- ^ Nikiforidis, G.; Xiang, Y.; Daoud, W.A. (2015). "Electrochemical behavior of carbon paper on cerium methanesulfonate electrolytes for zinc-cerium flow battery". Electrochimica Acta. 157: 274–281. doi: 10.1016/j.electacta.2014.11.134.
- ^ Xie, Z.; Yang, B.; Yang, L.; Xu, X.; Cai, D.; Chen, J.; Chen, Y.; He, Y.; Li, Y.; Zhou, X. (2015). "Addition of graphene oxide into graphite toward effective positive electrode for advanced zinc-cerium redox flow battery". Journal of Solid State Electrochemistry. 19 (11): 3339–3345. doi: 10.1007/s10008-015-2958-9. S2CID 93129998.
- ^ Na, Z.; Xu, S.; Yin, D.; Wang, L (2015). "A cerium–lead redox flow battery system employing supporting electrolyte of methanesulfonic acid". Journal of Power Sources. 295: 28–32. doi: 10.1016/j.jpowsour.2015.06.115.
- ^ Nikiforidis, G.; Daoud, W.A. (2015). "Indium modified graphite electrodes on highly zinc containing methanesulfonate electrolyte for zinc-cerium redox flow battery". Electrochimica Acta. 168: 394–402. doi: 10.1016/j.electacta.2015.03.118.
- ^ Arenas, L.F.; Walsh, F.C.; de Leon, C. (2015). "The importance of cell geometry and electrolyte properties to the cell potential of Zn-Ce hybrid flow batteries". Journal of the Electrochemical Society. 163 (1): A5170–A5179. doi: 10.1149/2.0261601jes.
- ^ Walsh, Frank C.; Ponce de Léon, Carlos; Berlouis, Len; Nikiforidis, George; Arenas-Martínez, Luis F.; Hodgson, David; Hall, David (2014). "The Development of Zn-Ce Hybrid Redox Flow Batteries for Energy Storage and Their Continuing Challenges" (PDF). ChemPlusChem. 80 (2): 288–311. doi: 10.1002/cplu.201402103.
- ^ Arenas, L.F.; Ponce de León, C.; Walsh, F.C. (2016). "Electrochemical redox processes involving soluble cerium species" (PDF). Electrochimica Acta. 205: 226–247. doi: 10.1016/j.electacta.2016.04.062.
