| |

| |
| Identifiers | |
|---|---|
3D model (
JSmol)
|
|
| ChemSpider | |
| |
| |
| Properties | |
| XeOF4 | |
| Molar mass | 223.23 g/mol |
| Appearance | colorless liquid |
| Density | 3.17 g/cm3, liquid |
| Melting point | −46.2 °C (−51.2 °F; 227.0 K) |
| Reacts with water | |
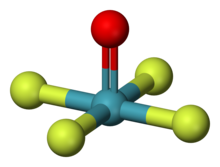
| Structure | |
| square pyramidal [1] [2] | |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Xenon oxytetrafluoride (
Xe
O
F
4) is an
inorganic
chemical compound. It is an unstable colorless liquid
[2]
[3] with a melting point of −46.2 °C (−51.2 °F; 227.0 K)
[4] that can be synthesized by partial
hydrolysis of
XeF
6, or the reaction of
XeF
6 with
silica
[3] or
NaNO
3:
[5]
A high-yield synthesis proceeds by the reaction of XeF
6 with
POF
3 at −196 °C (−320.8 °F; 77.1 K).
[6]
Like most xenon oxides, it is extremely reactive, and it hydrolyses in water to give hazardous and corrosive products, including hydrogen fluoride:
In addition, some ozone and fluorine is formed.
Reactions
XeOF
4 reacts with H
2O in the following steps:
The
XeO
3 formed is a dangerous explosive, decomposing explosively to Xe and O
2:
- 2 XeO
3 → 2 Xe + 3 O
2
In its liquid form, XeOF
4 exhibits amphoteric behaviour, forming complexes with both strong Lewis bases like
CsF and strong Lewis acids like
SbF
5.
[7] It forms a 1:1 adduct with
XeF
2, isostructural with XeF
2·IF
5,
[8] as well as various heavy
alkali metal fluorides.
[4]
The reaction of XeOF
4 with
XeO
3 provides a convenient synthesis route for
XeO
2F
2.
[9]
External links
- Xenon tetrafluoride oxide in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD) (retrieved 2022-04-13)
References
- ^ Martins, Joseph; Wilson, E. Bright Jr. (1964). "Microwave Spectrum of Xenon Oxytetrafluoride". The Journal of Chemical Physics. 41 (570): 570–571. Bibcode: 1964JChPh..41..570M. doi: 10.1063/1.1725910. ISSN 0021-9606.
- ^ a b Smith, D. F. (1963-05-24). "Xenon Oxyfluoride". Science. 140 (3569): 899–900. Bibcode: 1963Sci...140..899S. doi: 10.1126/science.140.3569.899. ISSN 0036-8075. JSTOR 00368075. LCCN 17024346. OCLC 1644869. PMID 17810680. S2CID 42752536.
- ^ a b Ibers, James A. (October 1965). "Molecular Structure". Annual Review of Physical Chemistry. 16: 375–396. Bibcode: 1965ARPC...16..375I. doi: 10.1146/annurev.pc.16.100165.002111. ISSN 0066-426X. LCCN a51001658. OCLC 1373069.
- ^ a b Selig, Henry (1966-02-01). "Complexes of Xenon Oxide Tetrafluoride". Inorganic Chemistry. 5 (2): 183–186. doi: 10.1021/ic50036a004. ISSN 0020-1669.
- ^ Christe, Karl O.; Wilson, William W. (April 1988). "Convenient synthesis of xenon oxide tetrafluoride". Inorganic Chemistry. 27 (7): 1296–1297. doi: 10.1021/ic00280a043. ISSN 0020-1669.
- ^ Nielsen, Jon B.; Kinkead, Scott A.; Eller, P. Gary (1990-09-01). "A New Synthesis of Xenon Oxytetrafluoride, XeOF4". Inorganic Chemistry. 29 (18): 3621–3622. doi: 10.1021/ic00343a063. ISSN 0020-1669.
- ^ Martin-Rovet, D.; Angelié, C.; Cauchetier, M.; Schrobilgen, G. J. (September 1982). "Various aspects of the reactivity of the xenon(VI) oxyfluoride: XeOF4". Journal of Fluorine Chemistry. 21 (1): 10. doi: 10.1016/S0022-1139(00)85330-0. ISSN 0022-1139.
- ^ Bartlett, N.; Wechsberg, M. (October 1971). "The Xenon Difluoride Complexes XeF2 · XeOF4; XeF2 · XeF6 · AsF5 and XeF2 · 2 XeF6 · 2 AsF5 and Their Relevance to Bond Polarity and Fluoride Ion Donor Ability of XeF2 and XeF6". Zeitschrift für anorganische und allgemeine Chemie. 385 (1): 5–17. doi: 10.1002/zaac.19713850103. ISSN 0044-2313. OCLC 1770423.
- ^ Huston, John L. (September 1967). "Xenon dioxide difluoride: isolation and some properties". The Journal of Physical Chemistry A. 71 (10): 3339–3341. doi: 10.1021/j100869a035. ISSN 1089-5639.