HAEMOCHROMATOSIS Information
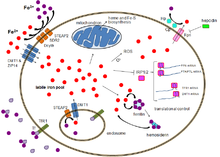
Haemochromatosis which is also known as IRON OVERLOAD, is a inherited disorder where excessive iron absorption due to defective iron metabolism, leads to increased body iron storage with deposition of iron in parenchymal cells of the liver, heart, pancreas and other organs. Iron metabolism is the set of chemical reactions maintaining human homeostasis of iron at both the systemic and cellular level. It is important to understand iron metabolism in order to understand proper cause of hemochromatosis.
Types
- Hereditary haemochromatosis
The genes that cause hemochromatosis are inherited, but only a minority of people who have the genes ever develop serious problems. Signs and symptoms of hereditary hemochromatosis usually appear in midlife.A number of genes have been implicated in this disorder and at present, mutations in five different genes are known to cause iron overload.
2. Secondary/Acquired Haemochromatosis
This type of hemochromatosis is caused due to other diseases and disorders like thalassemia, Cirrhosis, anemia and even problems such as alcohol abuse . Blood transfusions can also cause it.
3.Juvenile Haemochromatosis
Juvenile haemochromatosis or haemochromatosis type 2 is a rare autosomal recessive disorder which causes iron overload at a young age, affects both sexes equally and is characterized by a prevalence of hypogonadism and cardiopathy.Iron starts to accumulate in early age and symptoms start appearing in childhood and adolescence rather than in adulthood as is the case in hereditary hemochromatosis.
4.Neonatal Haemochromatosis
Neonatal hemochromatosis is not a single disorder but is a syndrome with an unclear etiology. Neonatal hemochromatosis represents disordered iron handling due to injury to the perinatal liver and can be thought of as a form of fulminant hepatic failure.
Causes
A gene called HFE is most often the cause of hereditary hemochromatosis. The HFE gene has two common mutations C282Y and H63D. Homozygosity for C282Y is present in 60-90% of patients with HFE1. A minority of cases will have one gene with the C282Y mutation, and the other with a histidine to aspartate mutation at amino acid 63 (H63D mutation) – this pattern is described as compound heterozygosity. Only a very small proportion of compound heterozygotes develop clinically significant iron overload, and then the level of iron loading is usually less than seen in C282Y homozygotes. Most C282Y heterozygotes (one mutation only) express minor or no abnormalities of serum iron indices but a few develop progressive iron-overload and overt disease. H63D homozygotes and heterozygotes do not develop clinically significant iron overload. Other HFE mutations (S65C) do not cause clinically significant iron overload.
Haemochromatosis type 2 is unrelated to HFE and the corresponding locus (named HFE2) maps to the long arm of chromosome 1, at 1q21.
Recently, other types of haemochromatosis have been characterized. Haemochromatosis type 3 is caused by mutations in transferrin receptor 2 (TFR2) gene. Haemochromatosis type 4 is an a typical form of haemochromatosis characterized by dominant inheritance, increased serum ferritin, but normal transferrin saturation and iron overload in macrophages and phagocytic cells due to mutation in the ferroportin 1 (FP1) gene.
secondary haemochromatosis can be caused by excessive alcohol consumption,Severe chronic haemolysis of any cause,uptake of excessive iron supplements and Multiple frequent blood transfusions.
Symptoms
Many patients will have no symptoms or signs suggestive of this disorder. Males are more likely to suffer from this disorder than females as they lose iron through menstrual flow until menopause,hence protected from the disorder.
Common symptoms include:
- Joint pain or Arthritis, from calcium pyrophosphate deposition in joints. The most commonly affected joints are those of the hands, particularly the knuckles of the second and third fingers.[6]
- Abdominal pain
- Fatigue
- Weakness Later signs and symptoms of the disease may include:
- Diabetes due to iron depostion in the pancreatic islet beta cells leading to cell death.
- Loss of sex drive and can also be the reason for erectile dysfunction
- Impotence due to testicular atrophy
- Heart failure
- Liver failure
- grey skin pigmentation known as "bronzing of skin".
Diagnosis

- Physical examination : swelling of the liver or spleen or any such indicator and also skin color changes.
- Screening test : The transferrin saturation and serum ferritin concentration are the most useful initial tests for haemochromatosis in people without a family history.a serum ferritin value of over 300 ng/mL (670 pmol/L) indicates iron overload in males and over 150 or 200ng/mL (330 or 440 pmol/L) in female after menopause. Transferrin saturation values greater than 45% indicates iron overload.
- Liver biopsy was previously the gold standard in the diagnosis of haemochromatosis. This allows histological staining of iron and measurement of hepatic iron concentration. Haemochromatosis can usually be confidently diagnosed without liver biopsy. However, biopsy is recommended if there is suspicion of cirrhosis.
- DNA testing
Treatment
The treatment of haemochromatosis consists of life-long venesection therapy, which depletes the body of iron by removal of iron in haemoglobin. 500ml of blood contains approximately 250mg of iron.The objective is to remove the excess of iron from blood and tissue, the easiest and foremost way to do this is to by removing blood weekly—just like donating it, an amount is extracted weekly and in fact it is safe to donate as well. This process is known as Phlebotomy
It is rare for patients not to tolerate venesection therapy, but this may occur in patients with severe cardiac disease, anaemia or hypoproteinemia. These patients may be given chelation therapy (desferrioxamine) for removal of iron but this is costly and in practice is rarely needed.
The prognosis of haemochromatosis has been significantly improved by venesection therapy. Overall cumulative survival is 76% at 10 years and 49% at 20 years. Non-cirrhotic patients diagnosed and treated early have a normal life expectancy compared to age and sex-matched controls, provided they continue treatment.
References
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3940210/ [1]
http://membes.gesa.org.au/membes/files/Clinical%20Guidelines%20and%20Updates/Haemochromatosis.pdf [2]
http://www.healthresource4u.com/hemochromatosis-causes-pictures-symptoms-treatment.html [3]
http://onlinelibrary.wiley.com/doi/10.1046/j.1365-2141.2002.03509.x/full
-
^ Feldman, Amy G.; Whitington, Peter F. (2017-03-14).
"Neonatal Hemochromatosis". Journal of Clinical and Experimental Hepatology. 3 (4): 313–320.
doi:
10.1016/j.jceh.2013.10.004.
ISSN
0973-6883.
PMC
3940210.
PMID
25755519.
{{ cite journal}}: CS1 maint: PMC format ( link) - ^ "Parallels Plesk Automation 11.5". membes.gesa.org.au. Retrieved 2017-03-14.
- ^ "Hemochromatosis - Causes, Pictures, Symptoms And Treatment". Health Resource. 2013-11-01. Retrieved 2017-03-14.