| |

| |
| Names | |
|---|---|
|
Preferred IUPAC name
1,2,3,4-Tetrahydronaphthalene | |
| Other names
1,2,3,4-Tetrahydronaphthalene, Benzocyclohexane, NSC 77451, Tetrahydronaphthalene, Tetranap
| |
| Identifiers | |
3D model (
JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.946 |
| KEGG | |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C10H12 | |
| Molar mass | 132.206 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.970 g/cm3 |
| Melting point | −35.8 °C (−32.4 °F; 237.3 K) |
| Boiling point | 206 to 208 °C (403 to 406 °F; 479 to 481 K) |
| Insoluble | |
| Viscosity | 2.02 cP at 25 °C [1] |
| Hazards | |
| Flash point | 77 °C (171 °F; 350 K) |
| 385 °C (725 °F; 658 K) | |
| Safety data sheet (SDS) | JT Baker MSDS |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
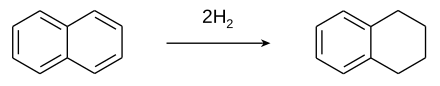
Tetralin (1,2,3,4-tetrahydronaphthalene) is a hydrocarbon having the chemical formula C10H12. It is a partially hydrogenated derivative of naphthalene. It is a colorless liquid that is used as a hydrogen-donor solvent. [2]
Production
Tetralin is produced by the catalytic hydrogenation of naphthalene. [2]
Although nickel catalysts are traditionally employed, many variations have been evaluated. [3] Over-hydrogenation converts tetralin into decahydronaphthalene ( decalin). Rarely encountered is dihydronaphthalene ( dialin).
Laboratory methods
In a classic named reaction called the Darzens tetralin synthesis, named for Auguste Georges Darzens (1926), derivatives can be prepared by intramolecular electrophilic aromatic substitution reaction of a 1-aryl-pent-4-ene using concentrated sulfuric acid, [4]

Uses
Tetralin is used as a hydrogen-donor solvent, for example in coal liquifaction. It functions as a source of H2, which is transferred to the coal. The partially hydrogenated coal is more soluble. [5] [2]
It has been used in sodium-cooled fast reactors as a secondary coolant to keep sodium seals around pump impellers solidified; however its use has been superseded by NaK. [6]: 24:30
It is also used for the laboratory synthesis of hydrogen bromide:
- C10H12 + 4 Br2 → C10H8Br4 + 4 HBr
The facility of this reaction is in part a consequence of the moderated strength of the benzylic C-H bonds.
Safety
LD50 (rats, oral) is 2.68 g/kg. Tetralin induces methemoglobinemia. [2]
References
- ^ Gonçalves, F. A.; Hamano, K.; Sengers, J. V. (1989). "Density and viscosity of tetralin and trans-decalin". International Journal of Thermophysics. 10 (4): 845. Bibcode: 1989IJT....10..845G. doi: 10.1007/BF00514480. S2CID 119843498.
- ^ a b c d Collin, Gerd; Höke, Hartmut; Greim, Helmut (2003). "Naphthalene and Hydronaphthalenes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi: 10.1002/14356007.a17_001.pub2. ISBN 978-3527306732.
- ^ Krichko, A. A.; Skvortsov, D. V.; Titova, T. A.; Filippov, B. S.; Dogadkina, N. E. (1969). "Production of tetralin by the hydrogenation of naphthalene-containing fractions". Chemistry and Technology of Fuels and Oils. 5: 18–22. doi: 10.1007/BF00727949. S2CID 95026822.
- ^ Michael B. Smith (2011). Organic Synthesis (third ed.). Academic Press. pp. 1209–1210. ISBN 9780124158849.
-
^ Isa, Khairuddin Md.; Abdullah, Tuan Amran Tuan; Md. Ali, Umi Fazara (2018). "Hydrogen donor solvents in liquefaction of biomass: A review". Renewable & Sustainable Energy Reviews. 81(Part_1): 1259–1268.
doi:
10.1016/j.rser.2017.04.006.
{{ cite journal}}: CS1 maint: multiple names: authors list ( link) - ^ US Atomic Energy Commission (1961) SRE Core Recovery Remediation method after a failure in the moderator cans due to a crack in the secondary coolant tubes in the SRE, Spring 1959. This caused a leak of Tetralin into the reactor.