| |
| Names | |
|---|---|
|
IUPAC name
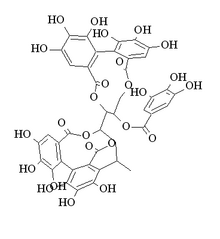
[(10R,11R)-10-[(15S,19S)-19-[2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-chromen-8-yl]-2,3,4,7,8,9-hexahydroxy-12,17-dioxo-13,16-dioxatetracyclo[13.3.1.05,18.06,11]nonadeca-1,3,5(18),6,8,10-hexaen-14-yl]-3,4,5,17,18,19-hexahydroxy-8,14-dioxo-9,13-dioxatricyclo[13.4.0.02,7]nonadeca-1(19),2,4,6,15,17-hexaen-11-yl] 3,4,5-trihydroxybenzoate
| |
| Identifiers | |
3D model (
JSmol)
|
|
PubChem
CID
|
|
| |
| |
| Properties | |
| C42H30O25 | |
| Molar mass | 934,64 g/mol |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Stenophyllanin A is an ellagitannin. It can be found in Cowania mexicana, Coleogyne ramosissima [1] and Quercus stenophylla. [2]
References
- ^ Anti-tumor promoting activity of polyphenols from Cowania mexicana and Coleogyne ramosissima. Hideyuki Ito, Masateru Miyake, Eisei Nishitani, Kazuko Mori, Tsutomu Hatano, Takuo Okuda, Takao Konoshima, Midori Takasaki, Mutsuo Kozuka, Teruo Mukainaka, Harukuni Tokuda, Hoyoku Nishino and Takashi Yoshida, Cancer Letters, Volume 143, Issue 1, 23 August 1999, Pages 5–13, doi: 10.1016/S0304-3835(99)00160-3
- ^ Tannins and related compounds part 26: isolation and structures of stenophyllanins A, B, and C, novel tannins from Quercus stenophylla, G. Nonaka, H. Nishimura, I. Nishioka, J. Chem. Soc. Perkin Trans. 1, 1985, pp. 163–172, doi: 10.1039/P19850000163