| Octreotide scan | |
|---|---|
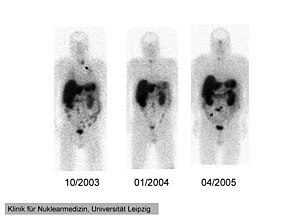 111In-pentetreotide scintigraphy of a 41-year-old man with
ectopic
Cushing's syndrome caused by a
neuroendocrine carcinoma of the
mesentery. Radiotracer accumulation in the left
thyroid in 10/2003 (arrow). The mesenterial neuroendocrine tumor became clearly visible in 4/2005 (arrow). | |
| Synonyms | ocreoscan |
| ICD-9-CM | 92.18 |
| OPS-301 code | 3-70c |
An octreotide scan is a type of SPECT scintigraphy used to find carcinoid, pancreatic neuroendocrine tumors, and to localize sarcoidosis. It is also called somatostatin receptor scintigraphy (SRS). Octreotide, a drug similar to somatostatin, is radiolabeled with indium-111, [1] and is injected into a vein and travels through the bloodstream. The radioactive octreotide attaches to tumor cells that have receptors for somatostatin (i.e. gastrinoma, glucagonoma, etc.). A gamma camera detects the radioactive octreotide, and makes pictures showing where the tumor cells are in the body, typically by a SPECT technique. A technetium-99m based radiopharmaceutical kit is also available. [2] [3]
Octreotide scanning is reported to have a sensitivity between 75% and 100% for detecting pancreatic neuroendocrine tumors. [4]
Instead of gamma-emitting 111In, certain octreotide derivatives such as edotreotide (DOTATOC) or DOTATATE are able to be linked by chelation to positron-emitting isotopes such as gallium-68 and copper-64 which in turn can be evaluated with more precise (compared with SPECT) scanning techniques such as PET-CT. Thus, the octreotide scan is now being replaced in most centers with gallium-68 DOTATATE and copper-64 DOTATATE scans. Somatostatin receptor imaging can now be performed with positron emission tomography (PET) which offers higher resolution and more rapid imaging. [5]
Indications
An octreotide scan may be used to locate suspected primary neuroendocrine tumours (NET) or for follow-up or staging after treatment. [6] [7] [8]
Where indicated, octreotide scanning for NET tumors is being increasingly replaced by gallium-68 DOTA and copper-64 DOTATATE scans. [9]
Procedure
Indium-111
 | |
| Clinical data | |
|---|---|
| Trade names | Octreoscan |
| Other names | MP-1727 |
| License data | |
|
Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| UNII | |
| Chemical and physical data | |
| 3D model ( JSmol) | |
| |
| |
The indium-111 pentetreotide radiopharmaceutical is prepared from a kit in a radiopharmacy. Pentetreotide is a DTPA conjugate of octreotide. [6] [11]
Approximately 200 megabecquerels (MBq) of indium-111 is injected intravenously. Imaging takes place 24 hours after injection, but may also be carried out at 4 and 48 hours. [7] [12]
Technetium-99m
The 99mTc product is supplied as a kit with two vials, one containing the chelating agent ethylenediaminediacetic acid (EDDA) and the other the HYNIC-Tyr3-octreotide chelator and somatostatin analog. [13] Approximately 400-700 MBq may be administered, with imaging at 2, 4, and occasionally 24 hours post administration. [14] 99mTc based octreotide imaging shows slightly higher sensitivity than 111In. [2] [15]
References
- ^ medicinenet.com > Carcinoid Syndrome (cont.) By Dennis Lee and Jay Marks. Retrieved Mars 2011
- ^ a b Briganti V, Cuccurullo V, Berti V, Di Stasio GD, Linguanti F, Mungai F, Mansi L (30 November 2020). "99mTc-EDDA/HYNIC-TOC is a New Opportunity in Neuroendocrine Tumors of the Lung (and in other Malignant and Benign Pulmonary Diseases)". Current Radiopharmaceuticals. 13 (3): 166–176. doi: 10.2174/1874471013666191230143610. PMC 8193811. PMID 31886756.
- ^ Garai I, Barna S, Nagy G, Forgacs A (November 2019). "Limitations and pitfalls of 99mTc-EDDA/HYNIC-TOC (Tektrotyd) scintigraphy". Nuclear Medicine Review. Central & Eastern Europe (in German). 19 (2): 93–98. doi: 10.1007/s00117-019-0574-x. PMID 27479887. S2CID 199443705.
- ^ Kwekkeboom DJ, Krenning EP (April 2002). "Somatostatin receptor imaging". Seminars in Nuclear Medicine. 32 (2): 84–91. doi: 10.1053/snuc.2002.31022. PMID 11965603.
- ^ Hofman MS, Kong G, Neels OC, Eu P, Hong E, Hicks RJ (February 2012). "High management impact of Ga-68 DOTATATE (GaTate) PET/CT for imaging neuroendocrine and other somatostatin expressing tumours". Journal of Medical Imaging and Radiation Oncology. 56 (1): 40–47. doi: 10.1111/j.1754-9485.2011.02327.x. PMID 22339744. S2CID 21843609.
- ^ a b Kwekkeboom DJ, Krenning EP, Scheidhauer K, Lewington V, Lebtahi R, Grossman A, et al. (2009). "ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: somatostatin receptor imaging with (111)In-pentetreotide". Neuroendocrinology. 90 (2): 184–189. doi: 10.1159/000225946. PMID 19713709. S2CID 13519061.
- ^ a b Bombardieri E, Ambrosini V, Aktolun C, Baum RP, Bishof-Delaloye A, Del Vecchio S, et al. (July 2010). "111In-pentetreotide scintigraphy: procedure guidelines for tumour imaging". European Journal of Nuclear Medicine and Molecular Imaging. 37 (7): 1441–1448. CiteSeerX 10.1.1.609.1835. doi: 10.1007/s00259-010-1473-6. PMID 20461371. S2CID 398563.
- ^ Saha GB (2010). Fundamentals of Nuclear Pharmacy. Springer. p. 145. ISBN 9781441958600.
- ^ Scott AT, Howe JR (August 2018). "Management of Small Bowel Neuroendocrine Tumors". Journal of Oncology Practice. 14 (8): 471–482. doi: 10.1200/JOP.18.00135. PMC 6091496. PMID 30096273.
- ^ "Octreoscan- indium in -111 pentetreotide kit". DailyMed. Retrieved 27 December 2021.
- ^ "In 111 pentetreotide". NCI Drug Dictionary. National Cancer Institute. 2011-02-02. Retrieved 3 October 2017.
- ^ Balon HR, Brown TL, Goldsmith SJ, Silberstein EB, Krenning EP, Lang O, et al. (December 2011). "The SNM practice guideline for somatostatin receptor scintigraphy 2.0". Journal of Nuclear Medicine Technology. 39 (4): 317–324. doi: 10.2967/jnmt.111.098277. PMID 22068564.
- ^ "TEKTROTYD". ROTOP Pharmaka. Retrieved 9 January 2022.
- ^ Garai I, Barna S, Nagy G, Forgacs A (2016). "Limitations and pitfalls of 99mTc-EDDA/HYNIC-TOC (Tektrotyd) scintigraphy". Nuclear Medicine Review. Central & Eastern Europe. 19 (2): 93–98. doi: 10.5603/NMR.2016.0019. PMID 27479887.
- ^ Federal Institute for Drugs and Medical Devices (6 May 2020). "TEKTROTYD Public Assessment Report (PAR)". Human MRIndex. Heads of Medicines Agencies. Retrieved 9 January 2022.
External links
- "Indium In 111 pentetreotide". Drug Information Portal. U.S. National Library of Medicine.
- Octreotide scan entry in the public domain NCI Dictionary of Cancer Terms
![]() This article incorporates
public domain material from
Dictionary of Cancer Terms.
U.S. National Cancer Institute.
This article incorporates
public domain material from
Dictionary of Cancer Terms.
U.S. National Cancer Institute.