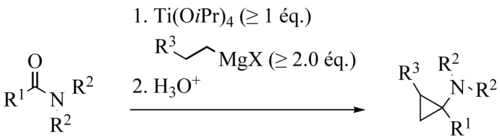| Kulinkovich reaction | |
|---|---|
| Named after | Oleg Kulinkovich |
| Reaction type | Ring forming reaction |
| Identifiers | |
| Organic Chemistry Portal | kulinkovich-reaction |
| RSC ontology ID | RXNO:0000682 |
The Kulinkovich reaction describes the organic synthesis of substituted cyclopropanols through reaction of esters with dialkyldialkoxytitanium reagents, which are generated in situ from Grignard reagents containing a hydrogen in beta-position and titanium(IV) alkoxides such as titanium isopropoxide. [1] This reaction was first reported by Oleg Kulinkovich and coworkers in 1989. [2]

Titanium catalysts are ClTi(OiPr)3 or Ti(OiPr)4, ClTi(OtBu)3 or Ti(OtBu)4, Grignard reagents are EtMgX, PrMgX or BuMgX. Solvents can be Et2O, THF, toluene. Tolerated Functional Groups: Ethers R–O–R, R–S–R, Imines RN=CHR. Amides, primary and secondary amines. Carbamates typically do not tolerate the reaction conditions, but tert-butyl carbamates (N-Boc derivatives) survive the transformation.
An asymmetric version of this reaction is also known with a TADDOL-based catalyst. [1]
Reaction mechanism
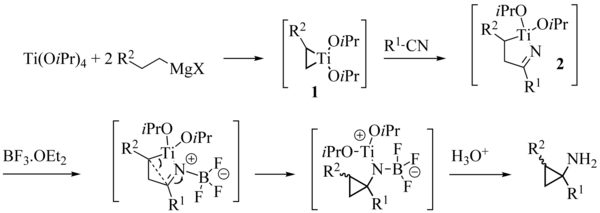
The generally accepted reaction mechanism initially utilizes two successive stages of transmetallation of the committed Grignard reagent, leading to an intermediate dialkyldiisopropoxytitanium complex. This complex undergoes a dismutation to give an alkane molecule and a titanacyclopropane 1. The insertion of the carbonyl group of the ester in the weakest carbon-titanium bond, leads to an oxatitanacyclopentane 2 being rearranged to ketone 3. Lastly, the insertion of the carbonyl group of 3 in the residual carbon-titanium connection forms a cyclopropane ring. In the transition state of this elementary stage, which is the limiting stage of the reaction, an agostic interaction stabilizing between the beta hydrogen and the R2 group and the titanium atom was called upon to explain the diastereoselectivity observed. Complex 4 obtained is a tetraalkyloxytitanium compound able to play a part similar to that of the starting tetraisopropyloxytitanate, which closes the catalytic cycle. At the end of the reaction, the product is mainly in the shape of the magnesium alcoholate 5, giving the cyclopropanol after hydrolysis by the reaction medium.
The step leading to the titanacyclopropane has been scrutinized computationally. Although the dialkyldiisopropoxytitanium complex has been proposed to undergo β hydrogen elimination followed by C–H reductive elimination to give the alkane and 1, it was found that β hydrogen abstraction by the alkyl group, leading directly to products without the intermediate titanium hydride, is a more favorable process. [3]
In broad strokes, and in a formal retrosynthetic sense, titanacyclopropane 1 behaves like a 1,2-dianion which adds into the ester twice: after the first addition into the ester, the resultant tetrahedral intermediate 2 collapses to give β-titanio ketone 3, which undergoes a second intramolecular addition to give the titanium salt of the cyclopropanol (4). (This species then undergoes transmetalation with Grignard reagent to regenerate 1 and close the catalytic cycle and give the product in the form of the magnesium salt (5).)
The reaction mechanism has been the subject of theoretical analysis. [4] Certain points remain nevertheless obscure. Intermediate titanium complexes of the ate type have been proposed by Kulinkovich. [5]
Ligand exchange with olefins
In 1993, the team of Kulinkovich highlighted the aptitude of the titanacyclopropanes to undergo ligand exchange with olefins. [6] This discovery was important, because it gave access to cyclopropanols more functionalized by making economic use of the Grignard of which normally at least two equivalents should have been engaged to obtain good yields. Cha and its team introduced the use of cyclic Grignard reagents, particularly adapted for these reactions. [7]
The methodology has been extended to intramolecular reactions [8]
De Meijere variation
| Kulinkovich–De Meijere reaction | |
|---|---|
| Named after | Oleg Kulinkovich Armin de Meijere |
| Reaction type | Ring forming reaction |
| Identifiers | |
| Organic Chemistry Portal | kulinkovich-demeijere-reaction |
| RSC ontology ID | RXNO:0000683 |
With amides instead of esters the reaction product is an aminocyclopropane in the De Meijere variation [9] [10]
The intramolecular reaction is also known: [11] [12] [13] [14] [15] [16] [17] [18] [19] [20]
Szymoniak variation
| Kulinkovich–Szymoniak reaction | |
|---|---|
| Named after | Oleg Kulinkovich Jan Szymoniak |
| Reaction type | Ring forming reaction |
| Identifiers | |
| Organic Chemistry Portal | kulinkovich-szymoniak-reaction |
| RSC ontology ID | RXNO:0000684 |
In the Szymoniak variation the substrate is a nitrile and the reaction product a cyclopropane with a primary amine group. [21] [22]
The reaction mechanism is akin the Kulinkovich reaction:
Additional reading
- Kulinkovich, O. G.; Sviridov, S. V.; Vasilevski, D. A. (1991). "Titanium(IV) Isopropoxide-Catalyzed Formation of 1-Substituted Cyclopropanols in the Reaction of Ethylmagnesium Bromide with Methyl Alkanecarboxylates". Synthesis. 1991 (3): 234. doi: 10.1055/s-1991-26431.
- Kulinkovich, O. G.; De Meijere, A. (2000). "1,n-Dicarbanionic Titanium Intermediates from Monocarbanionic Organometallics and Their Application in Organic Synthesis". Chem. Rev. 100 (8): 2789–834. doi: 10.1021/cr980046z. PMID 11749306.
- Sato, F.; Urabe, H.; Okamoto, S. (2000). "Synthesis of organotitanium complexes from alkenes and alkynes and their synthetic applications". Chem. Rev. 100 (8): 2835–86. doi: 10.1021/cr990277l. PMID 11749307.
- Wu, Y.-D.; Yu, Z.-X. (2001). "A theoretical study on the mechanism and diastereoselectivity of the Kulinkovich hydroxycyclopropanation reaction". J. Am. Chem. Soc. 123 (24): 5777–86. doi: 10.1021/ja010114q. PMID 11403612.
- Kulinkovich, O. G. (2004). "Alkylation of carboxylic acid derivatives with dialkoxytitanacyclopropane reagents". Russ. Chem. Bull. 53 (5): 1065–1086. doi: 10.1023/B:RUCB.0000041303.52400.ca. S2CID 98642553.
- Wolan, A.; Six, Y. (2010). "Synthetic transformations mediated by the combination of titanium(IV) alkoxides and grignard reagents: Selectivity issues and recent applications. Part 1: Reactions of carbonyl derivatives and nitriles". Tetrahedron. 66: 15–61. doi: 10.1016/j.tet.2009.10.050.
- Wolan, A.; Six, Y. (2010). "Synthetic transformations mediated by the combination of titanium(IV) alkoxides and Grignard reagents: Selectivity issues and recent applications. Part 2: Reactions of alkenes, allenes and alkynes". Tetrahedron. 66 (17): 3097–3133. doi: 10.1016/j.tet.2010.01.079.
- Corey, E. J.; Rao, S. A.; Noe, M. C. (1994). "Catalytic Diastereoselective Synthesis of Cis-1,2-Disubstituted Cyclopropanols from Esters Using a Vicinal Dicarbanion Equivalent". Journal of the American Chemical Society. 116 (20): 9345. doi: 10.1021/ja00099a068.
References
- ^ a b Cha, Jin Kun; Kulinkovich, Oleg G. (2012). "The Kulinkovich cyclopropanation of carboxylic acid derivatives". Organic Reactions. 77: 1–159. doi: 10.1002/0471264180.or077.01. ISBN 978-0471264187.
- ^ Kulinkovich, O. G.; Sviridov, S. V.; Vasilevskii, D. A.; Pritytskaya, T. S. (1989). "Reaction of ethylmagnesium bromide with carboxylic acid esters in the presence of tetraisopropoxytitanium". Zh. Org. Khim. 25: 2244.
- ^ Bertus, Philippe (11 November 2019). "From Dialkyltitanium Species to Titanacyclopropanes: An Ab Initio Study". Organometallics. 38 (21): 4171–4182. doi: 10.1021/acs.organomet.9b00509. ISSN 0276-7333.
-
^ Wu, Y.–D. and Yu, Z.-X. (2001). "A theoretical study on the mechanism and diastereoselectivity of the Kulinkovich hydroxycyclopropanation reaction". J. Am. Chem. Soc. 123 (24): 5777–86.
doi:
10.1021/ja010114q.
PMID
11403612.
{{ cite journal}}: CS1 maint: multiple names: authors list ( link) - ^ Kulinkovich, O. G.; Kananovich, D. G. (2007). "Advanced Procedure for the Preparation ofcis-1,2-Dialkylcyclopropanols – Modified Ate Complex Mechanism for Titanium-Mediated Cyclopropanation of Carboxylic Esters with Grignard Reagents". Eur. J. Org. Chem. 2007 (13): 2121–32. doi: 10.1002/ejoc.200601035.
- ^ Kulinkovich, O. G.; Savchenko, A. I.; Sviridov, S. V.; Vasilevski, D. A. (1993). "Titanium(IV) Isopropoxide-catalysed Reaction of Ethylmagnesium Bromide with Ethyl Acetate in the Presence of Styrene". Mendeleev Commun. 3 (6): 230–31. doi: 10.1070/MC1993v003n06ABEH000304.
- ^ Lee, J.; Kim, H.; Cha, J. K. (1996). "A New Variant of the Kulinkovich Hydroxycyclopropanation. Reductive Coupling of Carboxylic Esters with Terminal Olefins". J. Am. Chem. Soc. 118 (17): 4198–99. doi: 10.1021/ja954147f.
- ^ Kasatkin, A.; Sato, F. (1995). "Diastereoselective synthesis of trans-1,2-disubstituted cyclopropanols from homoallyl or bis-homoallyl esters via tandem intramolecular nucleophilic acyl substitution and intramolecular carbonyl addition reactions mediated by Ti(OPr-i)4 / 2 i-PrMgBr reagent". Tetrahedron Lett. 36 (34): 6079–82. doi: 10.1016/0040-4039(95)01208-Y.
- ^ Chaplinski, V.; De Meijere, A. (1996). "A Versatile New Preparation of Cyclopropylamines from Acid Dialkylamides". Angew. Chem. Int. Ed. 35 (4): 413–14. doi: 10.1002/anie.199604131.
- ^ De Meijere, A.; Winsel, H. and Stecker, B. Organic Syntheses, Vol. 81, p. 14 Archived 18 September 2012 at the Wayback Machine
- ^ Lee, J.; Cha, J. K. (1997). "Facile Preparation of Cyclopropylamines from Carboxamides". The Journal of Organic Chemistry. 62 (6): 1584. doi: 10.1021/jo962368d.
- ^ Chaplinski, V.; Winsel, H.; Kordes, M.; De Meijere, A. (1997). "A New Versatile Reagent for the Synthesis of Cyclopropylamines Including 4-Azaspiro[2.n]alkanes and Bicyclo[n.1.0]alkylamines". Synlett. 1997: 111–114. doi: 10.1055/s-1997-17828.
- ^ Cao, B.; Xiao, D.; Joullié, M. M. (1999). "Synthesis of Bicyclic Cyclopropylamines by Intramolecular Cyclopropanation of N-Allylamino Acid Dimethylamides". Organic Letters. 1 (11): 1799. doi: 10.1021/ol9910520. PMID 10814442.
- ^ Lee, H. B.; Sung, M. J.; Blackstock, S. C.; Cha, J. K. (2001). "Radical cation-mediated annulation. Stereoselective construction of bicyclo[5.3.0]decan-3-ones by aerobic oxidation of cyclopropylamines". Journal of the American Chemical Society. 123 (45): 11322–11324. doi: 10.1021/ja017043f. PMID 11697988.
- ^ Gensini, M.; Kozhushkov, S. I.; Yufit, D. S.; Howard, J. A. K.; Es-Sayed, M.; Meijere, A. D. (2002). "3-Azabicyclo[3.1.0]hex-1-ylamines by Ti-Mediated Intramolecular Reductive Cyclopropanation of α-(N-Allylamino)-Substituted N,N-Dialkylcarboxamides and Carbonitriles". European Journal of Organic Chemistry. 2002 (15): 2499. doi: 10.1002/1099-0690(200208)2002:15<2499::AID-EJOC2499>3.0.CO;2-V.
- ^ Tebben, G. D.; Rauch, K.; Stratmann, C.; Williams, C. M.; De Meijere, A. (2003). "Intramolecular Titanium-Mediated Aminocyclopropanation of Terminal Alkenes: Easy Access to Various Substituted Azabicyclo[n.1.0]alkanes1". Organic Letters. 5 (4): 483–485. doi: 10.1021/ol027352q. PMID 12583749.
- ^ Ouhamou, N.; Six, Y. (2003). "Studies on the intramolecular Kulinkovich?De Meijere reaction of disubstituted alkenes bearing carboxylic amide groups". Organic & Biomolecular Chemistry. 1 (17): 3007–9. doi: 10.1039/b306719j. PMID 14518121.
- ^ Gensini, M.; De Meijere, A. (2004). "Cyclopropane-Annelated Azaoligoheterocycles by Ti-Mediated Intramolecular Reductive Cyclopropanation of Cyclic Amino Acid Amides". Chemistry: A European Journal. 10 (3): 785–790. doi: 10.1002/chem.200305068. PMID 14767944.
- ^ Larquetoux, L.; Kowalska, J. A.; Six, Y. (2004). "The Formal [3+2+1] Cyclisation of Cyclopropylamines with Carboxylic Anhydrides: A Quick Access to Polysubstituted 2,3,3a,4-Tetrahydro6(5H)-indolone Ring Systems". European Journal of Organic Chemistry. 2004 (16): 3517. doi: 10.1002/ejoc.200400291.
- ^ Larquetoux, L.; Ouhamou, N.; Chiaroni, A. L.; Six, Y. (2005). "The Intramolecular Aromatic Electrophilic Substitution of Aminocyclopropanes Prepared by the Kulinkovich–De Meijere Reaction". European Journal of Organic Chemistry. 2005 (21): 4654. doi: 10.1002/ejoc.200500428.
- ^ Bertus, P.; Szymoniak, J. (2001). "New and easy route to primary cyclopropylamines from nitriles". Chemical Communications (18): 1792–1793. doi: 10.1039/b105293b. PMID 12240317.
- ^ Chaplinski, V.; De Meijere, A. (1996). "A Versatile New Preparation of Cyclopropylamines from Acid Dialkylamides". Angewandte Chemie International Edition in English. 35 (4): 413. doi: 10.1002/anie.199604131.