| |

| |
| Names | |
|---|---|
|
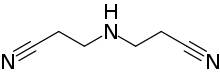
Preferred IUPAC name
3,3′-Azanediyldipropanenitrile | |
| Other names
Bis(2-cyanoethyl)amine
| |
| Identifiers | |
3D model (
JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.003.566 |
| EC Number |
|
PubChem
CID
|
|
| UNII | |
| UN number | 3334 |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C6H9N3 | |
| Molar mass | 123.159 g·mol−1 |
| Density | 1.02 |
| Melting point | −5.5 °C (22.1 °F; 267.6 K) |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
IDPN (3,3'-iminodipropanenitrile) is a neurotoxin with ototoxic and hepatotoxic effects. It causes irreversible movement disorder. [1] [2] [3]
Ototoxicity
IDPN has been shown to kill vestibular hair cells, disrupting normal vestibular function, in rats, [4] mice, guinea pigs, and frogs. [5] In rodents, the loss of vestibular function results in balance-related deficits, including circling behavior, retropulsion, and head bobbing, as well as weight loss. [5] Type I hair cells are more sensitive to IDPN toxicity than Type II hair cells. [4] No regeneration of vestibular hair cells was observed, thus these effects can be considered to be irreversible. [4]
IDPN has also been shown to kill cochlear hair cells, affecting auditory function. [6] IDPN-induced hearing loss covered a broad range of frequencies.
References
- ^ Sedó-Cabezón, Lara; Jedynak, Paulina; Boadas-Vaello, Pere; Llorens, Jordi (1 October 2015). "Transient alteration of the vestibular calyceal junction and synapse in response to chronic ototoxic insult in rats". Disease Models & Mechanisms. 8 (10): 1323–1337. doi: 10.1242/dmm.021436. PMC 4610239. PMID 26398945.
-
^ Khan, HA; Ibrahim, KE (12 October 2015).
"Pattern of neurobehavioral and organ-specific toxicities of β, β'-iminodipropionitrile in mice". Archives of Medical Science. 11 (5): 1137–44.
doi:
10.5114/aoms.2015.54871 (inactive 31 January 2024).
PMC
4624758.
PMID
26528360.
{{ cite journal}}: CS1 maint: DOI inactive as of January 2024 ( link) - ^ Ogata, Keiko; Kushida, Masahiko; Miyata, Kaori; Sumida, Kayo; Takeda, Shuji; Izawa, Takeshi; Kuwamura, Mitsuru; Yamate, Jyoji (2016). "Alteration of microRNA expressions in the pons and medulla in rats after 3,3′-iminodipropionitrile administration". Journal of Toxicologic Pathology. 29 (4): 229–236. doi: 10.1293/tox.2016-0019. PMC 5097965. PMID 27821907.
- ^ a b c Llorens, J.; Demêmes, D. (1994-06-01). "Hair cell degeneration resulting from 3,3'-iminodipropionitrile toxicity in the rat vestibular epithelia". Hearing Research. 76 (1–2): 78–86. doi: 10.1016/0378-5955(94)90090-6. ISSN 0378-5955. PMID 7928719. S2CID 4761312.
- ^ a b Soler-Martín, Carla; Díez-Padrisa, Núria; Boadas-Vaello, Pere; Llorens, Jordi (2007-03-01). "Behavioral Disturbances and Hair Cell Loss in the Inner Ear Following Nitrile Exposure in Mice, Guinea Pigs, and Frogs". Toxicological Sciences. 96 (1): 123–132. doi: 10.1093/toxsci/kfl186. ISSN 1096-6080. PMID 17159233.
- ^ Crofton, K. M.; Janssen, R.; Prazma, J.; Pulver, S.; Barone, S. (November 1994). "The ototoxicity of 3,3'-iminodipropionitrile: functional and morphological evidence of cochlear damage". Hearing Research. 80 (2): 129–140. doi: 10.1016/0378-5955(94)90104-X. ISSN 0378-5955. PMID 7896571. S2CID 4699179.