| Chlamydomonas nivalis | |
|---|---|
| |
|
Scientific classification
| |
| (unranked): | Viridiplantae |
| Division: | Chlorophyta |
| Class: | Chlorophyceae |
| Order: | Chlamydomonadales |
| Family: | Chlamydomonadaceae |
| Genus: | Chlamydomonas |
| Species: | C. nivalis
|
| Binomial name | |
| Chlamydomonas nivalis | |
| Synonyms [4] [5] [6] | |
Chlamydomonas nivalis, also referred to as Chloromonas typhlos, [2] [1] is a unicellular red-coloured photosynthetic green alga that is found in the snowfields of the alps and polar regions all over the world. They are one of the main algae responsible for causing the phenomenon of watermelon snow (also blood snow, raspberry snow [7]), where patches of snow appear red or pink. The first account of microbial communities that form red snow was made by Aristotle. Researchers have been active in studying this organism for over 100 years.
Although C. nivalis is closely related to Chlamydomonas reinhardtii, the environmental conditions each species inhabits are very different. C. nivalis can be found in mountains, snowfields, and polar regions around the world. The habitat of C. nivalis subjects the cells to environmental extremes including limited nutrients, low temperatures, and intense sunlight. In comparison with the mesophilic C. reinhardtii, C. nivalis has special mechanisms that allow it to be cryotolerant and survive on rock surfaces as well as in soil, meltwater, and snow. Secondary carotenoids, a thick cell wall, and particles on the cell wall are some characteristics that protect the cyst from light, drought, and radiation stress. Although the seasonal mobile to dormant life cycle of C. nivalis is complex, it also helps the algae exploit its niche and survive unfavourable conditions. As a result, C. nivalis is one of the best known and studied snow algae. When taking account of the photoprotective effect of its secondary carotenoid, astaxanthin, among the other adaptive mechanisms to its extreme habitat, it can be understood how C. nivalis became so dominant in microbial snow algae communities. Green motile offspring are produced in the spring and throughout the summer. They develop into red dormant cysts, the stage where this organism spends most of its life cycle, as the winter season begins and remain a cyst until the spring.
This alga is an interesting organism for researchers in various fields to study due to its possible role in lowering global albedo, ability to survive in extreme environments, and production of commercially relevant compounds. Additionally, its life cycle is still being studied today in an effort to better understand this organism and amend previous classification errors.
Etymology
The name Chlamydomonas nivalis is of compound Greek and Latin origin. Chlamydomonas is ultimately derived from the Ancient Greek χλαμύς (khlamús, “cloak, mantle”) and μονάς (monás, “solitary”), [8] while nivalis, from the Latin nivālis, translates to ‘found growing in or near snow’, as this species of algae are only found associated with snow or near snowy areas. [9] [10]
Description
The seasonal life cycle of C. nivalis can be broken down to three stages based on the colour of the cell as a result of carotenoid composition, which are green, orange, and red. [11] Orange cells and red cells are the most difficult to differentiate as they look similar while the red and green cells are easiest to differentiate as they have more significant differences in composition. [12] Cells at the red stage were previously described as a separate species than the green cells, but were later discovered to be different stages of the C. nivalis’ complex life cycle. [5]
Small green coloured motile cells of the young C. nivalis at the green stage are produced in spring or early summer when temperatures are warmer and zygotes undergo meiosis in meltwater pools. [13] The biflagellated cells are slightly oval and about 5-15 µm in diameter. [12] [14] In this asexually reproductive phase, the cells are sensitive to temperature and drought stress. They avoid unfavourable light and temperature by swimming in the snow until they reach more optimal conditions. [15] [13] [11] Chloroplasts of green cells are irregularly shaped. [11] The dominating pigment, chlorophyll, gives the cell its characteristic hue and facilitates maximum cell growth through light absorption. Secondary carotenoid concentrations are much lower at this stage as the cells need photosynthetically active radiation for energy and growth. [12] Cells in the green stage also have less organic and inorganic particles on their surface compared to mature cysts. [15]
Later in the season, when nitrogen and nutrients becomes limited and radiation stress increases, the green cells will develop into flagellated sexual gametes that mate and produce new zygotes that have lost their flagella and are capable of surviving the winter period. [16] [14] Transformation into the zygote, or hypnoblast, is characterized by the production and accumulation of reserve materials that include sugars and lipids as well as the formation of esterified secondary carotenoids. [15] The secondary carotenoids will turn the green zygotes orange as they accumulate in the area around the plastids of the cell to protect the zygotes from UV radiation. [15] [12] Orange and red spores can be seen throughout the summer. During this stage, the cell wall will also begin to thicken to help the cell tolerate freezing temperatures and UV light. [14] [17] In addition, the color of these pigments reduces albedo such that individual cells may melt nearby ice and snow crystals to access limiting nutrients and water in an otherwise unavailable frozen state. [18]
History
The earliest documentation of red snow was made by Aristotle. [19] While he recognized that something must be contributing to the odd colouration, red snow was also commonly mistaken as mineral deposits or pollen up until the early 1900s. [20] In 1819, samples of ‘red snow’ were brought back for examination with a returning Arctic expedition under Sir John Ross. The samples were sent to Robert Brown and Francis Bauer for examination. Both men came to different conclusions on how to classify the specimens. Brown believed the specimen to be a unicellular alga while Bauer declared it a new species of fungus, Uredo nivalis. [20] [4] [21] Over the next century, many researchers disputed over whether these organisms were lichen, plants, alga, or animal. It was not until the early 20th century when researchers finally began to agree on the algal nature of the organism and gave its currently known name, Chlamydomonas nivalis. [4] [21] In 1968 C. nivalis was officially recognized as a collective taxon. [22] Unfortunately, due to the lack of sequencing techniques, reliance on visually examining similarly looking snow alga, and complicated life cycle of this species, errors continued to be made in classifying this and other species of snow algae. Today, C. nivalis has become one of the most well-studied snow algae. Although its taxonomy is still being settled, the life cycle of this snow algae is now much better understood. [23] [4] [5] [24] The historical disputes about the classification and misclassification of specimens have resulted in a number of names from older publications that all mean to refer to C. nivalis. These are: Uredo nivalis, Sphaerella nivalis, Protococcus nivalis, and Haematococcus nivalis. [4] [5] [6]
Habitat and ecology
C. nivalis has been reported worldwide in mountainous regions, polar regions, or snowfields of every continent. [23] [25] It is the most abundant snow algae and typically composes the majority of cells identified in specimens taken from various sample sites. [15] Most habitats these algae reside in are very different from other species of the rest of the genus Chlamydomonas. [16] This includes, but is not limited to snow, rock surfaces, soil, meltwater, and cryoconite holes. [26] [27] [16] [28]
The environmental conditions C. nivalis is typically exposed to are considered to be extreme. The cells can experience low nutrient availability, acidity, intense sunlight, radiation, extreme temperature regimes, and darkness. [23] [13] [26] [29] Red-snow algae have been shown experimentally to be limited by both nutrients (N, P, and K) and liquid water. [30] C. nivalis spends the majority of its life in the cyst stage surrounded by snow at a depth that can range from 0–20 centimetres (0.0–7.9 in). [14] [31] This can change depending on if the cell is in a mobile stage and can move, the snow melts due to the onset of warm weather, or the onset of precipitation causes more snow to fall on the cells. [26] Cells that are exposed on unshaded snow may be subjected to high levels of visible light and ultraviolet radiation for an extended amount of time. Meanwhile, cells that are deep below the snow’s surface may experience darkness. [23] In its flagellated stage, the cell can move until it is in the most optimal position in the snow for moisture content, light, and temperature. [15] When in the immotile cyst stage, the C. nivalis cells must depend on the flow of meltwater to move it by chance to a favourable area. [26]
The temperatures in which this species can survive in ranges from below 0 °C to just above 20 °C. Growth is slow when temperatures are below 5 °C. At 5-15 °C the growth of C. nivalis cells can outperform the growth of C. reinhardtii cells. [27] Both species grow at the same rate at 20-25 °C. The growth of C. nivalis is suppressed when temperatures rise above 30 °C. [27] It is a true snow alga because it performs better in low temperatures than warm temperatures. [15] Due to C. nivalis’ ability to perform photosynthesis well from cold to moderate temperatures, this species is considered a cryotolerant mesophile rather than a cryophile. [6] [27] This organism is also very resilient as they can also survive in warm soil for weeks. They can also tolerate dryness and room temperature for as long as 6 months. [15]
Fungi, worms, bacteria, and viruses have been found to associate with or live in the same environment as C. nivalis. Encapsulated rod-shaped gram-negative bacteria have been found on the surface of C. nivalis cysts. The unknown bacteria were not detected in control samples that did not contain C. nivalis which strongly suggests that it must be associated with the algae. [32] Another bacterium, Mesorhizobium loti, was found as contamination in a C. nivalis culture, but further testing suggested that this bacteria may be synthesizing vitamin B12 for the algae. [33] In cryoconite holes C. nivalis can be found among bacteria, virus-like particles, ciliates, and Chlorophyte species. [28] Ice worms have also been found to live preferentially under C. nivalis in glaciers, possibly using the algae as a food source. [34] Infections of C. nivalis cells by chytrids, Chytridium chlamydococci, filamentous fungi, and Selenotila nivalis have also been observed. [6]
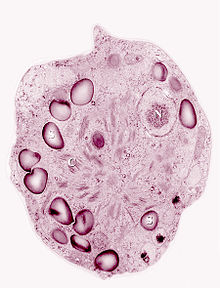
As winter approaches, the cells will approach the last stage of their life cycle. The orange cells mature into red cysts, the form in which it will remain for the remainder and longest portion of its life cycle. [26] [35] [14] Cells at this stage are most resistant to harsh environmental conditions. [15] Inorganic and organic materials such as bacteria, fungi, and dust particles coat the mucilage layer of the cell wall. [15] The inorganic impurities were found to be rich in silicon, iron, and aluminum. These elements can also be taken up into the cellular compartment and stored in vacuoles and may be an important source of mineral supply. [36] The cell wall, as the boundary that protects the inner contents of the cell from the harsh conditions in its habitat, is very rigid and hard to destroy. [15] It also may play a role in protecting the algal cells from desiccation during the freeze-thaw cycle alternations during seasonal changes. [32] The spherical immotile red cysts range from 35-40 µm in diameter. The cell contains one central chloroplast that has a naked pyrenoid, ribosomes, starch grains, and numerous small grana stacks composed of 3-7 thylakoids within it. [23] [15] [32] [37] Negatively charged phosphatidylglycerol composes the majority of the thylakoid membranes. [23] The thylakoid membrane lipid composition can also be changed to enhance lipid fluidity in response to lower temperatures. [27] An undulated membrane encloses the chloroplast. Lipid bodies and carotenoid globules surround the plastid. [37] A red secondary pigment, astaxanthin and esterified derivatives of it, accumulates up to 20 times the amount of chlorophyll a in the cytoplasmic lipid bodies of mature red spores. [23] [15] Astaxanthin protects the chloroplast from excessive light by absorbing a portion of it before it reaches the photosynthetic apparatus which subsequently prevents photoinhibition and UV damage. [35] The absorbed radiation is converted to heat, aiding in the melt of nearby snow and ice crystals to access needed nutrients and liquid water. [18] Astaxanthin can also act as a metabolic sink for the metabolically active spores that do not divide. [23] [17]
Within the cytoplasm there are several small cytoplasmic vacuoles with partially crystallized content within it. [36] While mitochondria are present, they are not very obvious. Most of the cytoplasmic space is taken up by the large plastid, lipid bodies, and carotenoid globules. [15] [32] C. nivalis has one centrally located nucleus that is also oriented such that it is covered by the carotenoid globules full of astaxanthin that will provide protection against UV radiation. [26] The majority (91%) of astaxanthin derivatives are stored in its monoester form within dormant C. nivalis red cysts. [23] [13] Astaxanthin is the pigment that makes the cell appear deep red. Other pigments that can also be found in C. nivalis include violaxanthin and adonirubin. [17]
Role in environmental processes and research
Visible algal blooms could be a crucial determinant of surface albedo. [38] It has been suggested that algal blooms partially composed of C. nivalis may contribute to lowering ice and snow albedo. [38] The red coloured pigments produced by the cell in combination with inorganic material could enhance the darkening over the snow and reduce the surface area of white snow. [39] Due to the absorption of solar energy by the alga, albedo would be reduced and the darker areas on the snow where the blooms form would melt more rapidly. [39] As a result, populations of C. nivalis would increase, creating a feedback loop that amplifies melting and reduces sunlight absorbance which contributes to glacier retreat and lowering albedo, as shown experimentally. [30] This is concerning to environmentalists and climate scientists. [40] [41] [42]
C. nivalis can be used as a model species for studying the cellular response mechanism to stressful conditions given the harsh conditions of its habitat. [43] It is also an important organism to study adaptation to extreme environments and may become one of the leading systems for research in cold adaptation. [5] C. nivalis is likely to have strong antioxidant capabilities, a robust repair mechanism, and other components that may be of interest to researchers. [26]
Thermophilic microalgae have gained biotechnological interest as a source for thermostable enzymes and commercial interest as a source for astaxanthin. [44] C. nivalis could also potentially be a source for pharmaceuticals, supplements, or beauty products if the algae could be mass produced for its astaxanthin. [45] [46] [47] The snow algae itself is likely safe to eat as there is no evidence supporting that it would cause diarrhea when ingested. [48]
References
- ^ a b Schoeters, Floris; Spit, Jornt; Azizah, Rahmasari Nur; Van Miert, Sabine (2022). "Pilot-Scale Cultivation of the Snow Alga Chloromonas typhlos in a Photobioreactor". Frontiers in Bioengineering and Biotechnology. 10: 896261. doi: 10.3389/fbioe.2022.896261. ISSN 2296-4185. PMC 9218667. PMID 35757813.
- ^ a b c "SAG 26.86 Chloromonas typhlos". sagdb.uni-goettingen.de.
- ^ Matsuzaki, Ryo; Hara, Yoshiaki; Nozaki, Hisayoshi (1 January 2012). "A taxonomic revision of Chloromonas reticulata (Volvocales, Chlorophyceae), the type species of the genus Chloromonas, based on multigene phylogeny and comparative light and electron microscopy". Phycologia. 51 (1): 74–85. doi: 10.2216/11-18.1. ISSN 0031-8884. S2CID 85094898.
- ^ a b c d e Sutton, E. A. (1970). “The physiology and life histories of selected cryophytes of the pacific Northwest”. Ph.D. Thesis. Oregon state university, Corvallis.
- ^ a b c d e Cvetkovska, M. C.; Hüner, N. P. A.; Smith, D. R. (2016). “Chilling out: the evolution and diversification of psychrophilic algae with a focus on Chlamydomonas”. Polar Biol. 40 (6): 1169-1184. doi: 10.1007/s00300-016-2045-4
- ^ a b c d Cepak, V.; Lukavsky, J. (2013) “Cryoseston of the Pirin Mountains, Bulgaria”. Acta Bot Croat. 72 (2): 257-268. doi: 10.2478/botcro-2013-0012.
- ^ "Blood snow invades an Antarctic island". 28 February 2020.
- ^ "Definition of CHLAMYDOMONAS".
- ^ "Charlton T. Lewis, Charles Short, A Latin Dictionary, nĭvālis".
- ^ Guiry, M. D. in Guiry, M.D. & Guiry, G.M. (2018). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; Accessed on: 04 March 2018.
- ^ a b c Lütz, C. (2012). “Plants in Alpine Regions”. Springer-Verlag Wien. doi: 10.1007/978-3-7091-0136-0
- ^ a b c d Beer, T.; Tanaka, Z.; Netzter, N.; Rothschild, L. J.; Chen, B. (2011). “An analysis of uncultured extremophilic snow algae by non-invasive single cell Raman spectroscopy”. Proc. SPIE 8152, Instruments, Methods, and Missions for Astrobiology XIV, 81520F. doi: 10.1117/12.896481
- ^ a b c d Bidigare, R. R.; Ondrusek, M. E.; Kennicutt II, M. C.; Iturriaga, R.; Harvey, H. R.; Hoham, H. W.; Macko, S. A. (1993). “Evidence for a photoprotective function for secondary carotenoids of snow algae”. J. Phycol. 29 (4): 427-434.
- ^ a b c d e Stibal, M.; Elster, J.; Sabacka, M.; Kastovska, K. (2007). “Seasonal and diel changes in photosynthetic activity of the snow alga Chlamydomonas nivalis (Chlorophyceae) from Svalbard determined by pulse amplitude modulation fluorometry”. FEMS Microbiology Ecology. 59 (2): 265–273. doi: 10.1111/j.1574-6941.2006.00264.x.
- ^ a b c d e f g h i j k l m n Remias, D.; Lütz-Meindl, U.; Lütz C. (2005). “Photosynthesis pigments and ultrastructure of the alpine snow alga Clamydomonas nivalis”. European Journal of Phycology. 40 (3): 259-268, doi: 10.1080/09670260500202148
- ^ a b c Müller, T.; Bleiss, W.; Martin, C.-D.; Rogaschewski, S.; Fuhr, G. (1998). “Snow algae from northwest Svalbard: their identification, distribution, pigment and nutrient content”. Polar Biology. 20 (1): 14-32.
- ^ a b c Remias, D.; Albert, A.; Lütz, L. (2010). "Effects of realistically simulated, elevated UV irradiation on photosynthesis and pigment composition of the alpine snow alga Chlamydomonas nivalis and the arctic soil alga Tetracystis sp. (Chlorophyceae)" (PDF). Photosynthetica. 48 (2): 269–277. doi: 10.1007/s11099-010-0033-4. S2CID 25291747.
- ^ a b Dial, R.; Ganey, G.; Skiles, M. (2018). “What color should glacier algae be? An ecological role for red carbon in the cryosphere”. FEMS Microbiology Ecology. 94 (3): fiy007. https://academic.oup.com/femsec/article/94/3/fiy007/4810544; Accessed on: 18 December 2018
- ^ Scientific American. Munn & Company. 1882-03-25. p. 181.
- ^ a b Clark, F. C. (1875) “Red Snow”. Am. Nat. 9:129–135.
- ^ a b Wille, N. (1903). “Algologische Notizen IX-XIV. Nytt” Magazin for Naturvidenskaberne. 41: 89-185.
- ^ Kol, E. (1968). “A note on red snow from New Zealand”. N. Z. J. Bot. 6 (2): 243-244. doi:10.1080/0028825X.1968.10429063
- ^ a b c d e f g h i Remias, D.; Pichrtova, M.; Pangratz, M.; Lütz, C.; Holzinger, A. (2016) “Ecophysiology, secondary pigments and ultrastructure of Chlainomonas sp. (Chlorophyta) from the European Alps compared with Chlamydomonas nivalis forming red snow”. FEMS Microbiol. Ecol. 92 (4). doi: 10.1093/femsec/fiw030
- ^ Brown, S. P.; Olson, B. J. S. C.; Jumpponen, A. (2015). “Fungi and algae co-occur in snow: an issue of shared habitat or algal facilitation of heterotrophs?”. Arct. Antarct. Alpine Res. 47 (4): 729–749. doi: 10.1657/AAAR0014-071
- ^ Uetake, J.; Yoshimura, Y.; Nagatsuka, N.; Kanda, H. (2012). “Isolation of oligotrophic yeasts from supraglacial environments of different altitude on the Gulkana Glacier (Alaska)”. FEMS Microbiol Ecol 82 (2): 279–286. doi: 10.1111/j.1574-6941.2012.01323.x.
- ^ a b c d e f g Gorton, H. L.; Vogelmann, T. C. (2003). “Ultraviolet radiation and the snow alga Chlamydomonas nivalis(Bauer) Wille”. Photochemistry and Photobiology. 77 (6): 608-615. doi: 10.1562/0031-8655(2003)0770608URATSA2.0.CO2
- ^ a b c d e Lukes, M.; Prochazkova, L.; Shmidt, V.; Nedbalova, L.; Kaftan, D. (2014). “Temperature dependence of photosynthesis and thylakoid lipid composition in the red snow alga Chlamydomonas cf. nivalis (Chlorophyceae)”. FEMS Microbiol. Ecol. 89 (2): 303-315. doi: 10.1111/1574-6941.12299
- ^ a b Säwström, C.; Mumford, P.; Marshall, W.; Hodson, A.; Laybourn-Parry, J. (2002). “The microbial communities and primary productivity of cryoconite holes in an Arctic glacier (Svalbard 79°N)”. Polar Biology. 25 (8): 591-596.
- ^ Leya, T.; Müller, T.; Ling, H. U.; Fuhr, G. (2004). “Snow algae from north-western Spitsbergen (Svalbard), Ber. Polarforsch. Meeresforsch. 492: 46-54.
- ^ a b Ganey, G.Q.; Loso, M.; Bryant Burgess, A.; Dial, R.J. (2017). “The role of microbes in snowmelt and radiative forcing on an Alaskan icefield”. Nature Geoscience. 10: 754-759. doi: 10.1038/NGEO3027
- ^ Mosser, J. L.; Mosser, A. G.; Brock. T. D. (1977). “Photosynthesis in the snow: the alga Chlamydomonas nivalis (Chlorophyceae)”. J. Phycol. 13 (1): 22-27. doi: 10.1111/j.1529-8817.1977.tb02881.x
- ^ a b c d Weiss, R. L. (1983). “Fine structure of the snow alga (Chlamydomonas nivalis) and associated bacteria”. J. Phycol. 19 (2): 200-204. doi: 10.1111/j.0022-3646.1983.00200.x
- ^ Kazamia, E.; Czesnick, H.; Nguyen, T. T.; Croft, M. T.; Sherwood, E.; Sasso, S.; Hodson, S. J.; Warren, M. J.; Smith, A. G. (2012). “Mutualistic interactions between vitamin B12 –dependent algae and heterotrophic bacteria exhibit regulation”. Environ. Microbiol. 14 (6): 1466-1476. doi: 10.1111/j.1462-2920.2012.02733.x.
- ^ Shain, D. H.; Mason, T. A.; Farrell, A. H.; Michalewicz, L. A. (2001). “Distribution and behaviour of ice worms (Mesenchytraeus solifugus) in south-central Alaska”. Canadian Journal of Zoology. 79 (10): 1813-1821. doi: 10.1139/z01-143
- ^ a b Williams, W. E.; Gorton, H. L.; Vogelmann, T. C. (2003). “Surface gas-exchange processes of snow algae”. Proc. Natl. Acad. Sci. USA. 100 (2): 562-566. doi: 10.1073/pnas.0235560100
- ^ a b Lütz-Meindl, U.; Lütz, C. (2006). “Analysis of element accumulation in cell wall attached and intracellular particles of snow algae by EELS and ESI”. Micron. 37 (5): 452-458.
- ^ a b Holzinger, A.; Lutz, C. (2006). “Algae and UV irradiation: Effects on ultrastructure and related metabolic functions”. Micron 37 (3): 190–207. doi: 10.1016/j.micron.2005.10.015.
- ^ a b Cook. J. M.; Hodson, A. J.; Taggart, A. J.; Mernild, S. H.; Tranter, M. (2017). “A predictive model for the spectral “bioalbedo” of 30 snow”. J. Geophys. Res. Earth Surf. 122 (1). doi:10.1002/2016JF003932, 2017.
- ^ a b Cook, J.M.; Hodson, A.; Gardner, A. S.; Flanner, M.; Tedstone, A. J.; Williamson, C.; et al (2017). “Quantifying bioalbedo: a new physically based model and discussion of empirical methods for characterising biological influence on ice and snow albedo”. The Cryosphere. 11: 2611-2632. doi: 10.5194/tc-11-2611-2017
- ^ Thomas, W. H.; Duval, B. (1995). “Sierra Nevada, California, U.S.A., snow algae: snow albedo changes, algal-bacterial interrelationships, and ultraviolet radiation effects”. Arct. Alp. Res. 27 (4): 389-399. doi: 10.2307/1552032
- ^ Hisakawa, N.; Quistad, S.D.; Hester, E.R.; Martynova, D.; Maughan, H.; Sala, E.; Rohwer, F. (2015). “Metagenomic and satellite analyses of red snow in the Russian Arctic”. PeerJ. 3, doi: 10.7717/peerj.1491.
- ^ Lutz, S.; Anesio, A. M.; Raiswell, R.; Edwards, A.; Newton, R. J.; Gill, F.; et al. (2016). “The biogeography of red snow microbiomes and their role in melting arctic glaciers”. Nat. Commun. 7. doi:11968 10.1038/ncomms11968
- ^ Wiencke, C.; Clayton, M.N. (2009). “Biology of polar benthic algae”. Bot. Mar. 52: 479–481. doi: 10.1515/BOT.2009.083
- ^ Varshney, P.; Mikulic, P.; Vonshak, A.; Beardall, J.; Wangikar, P.P. (2015). “Extremophilic micro-algae and their potential contribution in biotechnology”. Bioresour. Technol. 184: 363–372. doi: 10.1016/j.biortech.2014.11.040
- ^ Duval, B.; Shetty, K.; Thomas, W. H. (1999). “Phenolic compounds and antioxidant properties in the snow alga Chlamydomonas nivalis after exposure to UV light”. J. Appl. Phycol. 11: 559-566. doi: 10.1023/A:1008178208949
- ^ Martin, J. F.; Gudina, E.; Barredo, J. L. (2008). “Conversion of beta-carotene into astaxanthin: Two separate enzymes or a bifunctional hydroxylase-ketolase protein?”. Mirob. Cell. Fact. 20 (7): 3. doi: 10.1186/1475-2859-7-3.
- ^ Tominaga, M.; Beinlich, A.; Lima, E. A.; Tivey, M. A.; Hampton, B. A.; Weiss, B.; Harigane, Y. (2017). “Multi-scale magnetic mapping of serpentinite carbonation”. Nature Communications. 8: 1870-1880. doi:10.1038/s41467-017-01610-4
- ^ Fiore, D. C.; Mckee, D. D.; Janiga, M. A. (1997). “Red snow: is it safe to eat? A pilot study”. Wilderness Environ. Med. 8 (2): 94-95. doi: 10.1580/1080-6032(1997)008[0094:RSIIST] 2.3.CO;2