| |
| Names | |
|---|---|
|
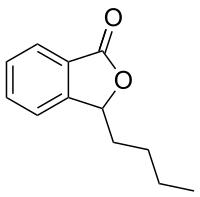
Preferred IUPAC name
3-Butyl-2-benzofuran-1(3H)-one | |
| Other names
3-Butylphthalide; 3-n-Butylphthalide
| |
| Identifiers | |
3D model (
JSmol)
|
|
| Abbreviations | NBP; BuPh |
| ChemSpider | |
| ECHA InfoCard | 100.025.455 |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C12H14O2 | |
| Molar mass | 190.242 g·mol−1 |
| Appearance | clear oily liquid |
| Related compounds | |
Related compounds
|
Phthalide |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Butylphthalide (3-n-butylphthalide or NBP) is one of the chemical constituents in celery oil, along with sedanolide, which is primarily responsible for the aroma and taste of celery. [1]
Studies in animal models suggest that butylphthalide may be useful for the treatment of hypertension [2] [3] and may have neuroprotective effects. [4] [5] [6] [7] In 2002, NBP was approved in China for the treatment of cerebral ischemia. [8]
NBP undergoes extensive metabolism in humans. [9] The major metabolites in human plasma were 3-OH-NBP, 10-OH-NBP, 10-CO-NBP, 11-COOH-NBP. The AUC of metabolites was much larger than that of NBP.
Minor side effects were observed in preclinical and clinical studies. The minor bioactivation pathway of NBP was proved to be mediated via sulfation of 3-OH-NBP. [10]
References
- ^ Wilson, Charles Welthy III (1970). "Relative recovery and identification of carbonyl compounds from celery essential oil". Journal of Food Science. 35 (6): 766–768. doi: 10.1111/j.1365-2621.1970.tb01989.x.
- ^ Houston, M (2005). "Nutraceuticals, Vitamins, Antioxidants, and Minerals in the Prevention and Treatment of Hypertension". Progress in Cardiovascular Diseases. 47 (6): 396–449. doi: 10.1016/j.pcad.2005.01.004. PMID 16115519.
- ^ D. Tsi and B. K. H. Tan (1997). "Cardiovascular Pharmacology of 3-n-butylphthalide in Spontaneously Hypertensive Rats". Phytotherapy Research. 11 (8): 576–582. doi: 10.1002/(SICI)1099-1573(199712)11:8<576::AID-PTR174>3.0.CO;2-7. S2CID 73033504.
- ^ Feng, X; Peng, Y; Liu, M; Cui, L (2011). "Dl-3-n-butylphthalide extends survival by attenuating glial activation in a mouse model of amyotrophic lateral sclerosis". Neuropharmacology. 62 (2): 1004–10. doi: 10.1016/j.neuropharm.2011.10.009. PMID 22056419. S2CID 37504365.
- ^ Zhang, T; Yan, W; Li, Q; Fu, J; Liu, K; Jia, W; Sun, X; Liu, X (2011). "3-n-butylphthalide (NBP) attenuated neuronal autophagy and amyloid-beta expression in diabetic mice subjected to brain ischemia". Neurological Research. 33 (4): 396–404. doi: 10.1179/1743132810Y.0000000006. PMID 21535939. S2CID 35636793.
- ^ Ji, XC; Zhao, WH; Cao, DX; Shi, QQ; Wang, XL (2011). "Novel neuroprotectant chiral 3-n-butylphthalide inhibits tandem-pore-domain potassium channel TREK-1". Acta Pharmacologica Sinica. 32 (2): 182–7. doi: 10.1038/aps.2010.210. PMC 4009930. PMID 21293470.
- ^ Xiong, N; Huang, J; Chen, C; Zhao, Y; Zhang, Z; Jia, M; Zhang, Z; Hou, L; Yang, H; Cao, Xuebing; Liang, Zhihou; Zhang, Yongxue; Sun, Shenggang; Lin, Zhicheng; Wang, Tao (2011). "Dl-3-n-butylphthalide, a natural antioxidant, protects dopamine neurons in rotenone models for Parkinson's disease". Neurobiology of Aging. 33 (8): 1777–91. doi: 10.1016/j.neurobiolaging.2011.03.007. PMID 21524431. S2CID 27840652.
- ^ Abdoulaye, I. A.; Guo, Y. J. (2016). "A Review of Recent Advances in Neuroprotective Potential of 3-N-Butylphthalide and Its Derivatives". BioMed Research International. 2016: 5012341. doi: 10.1155/2016/5012341. PMC 5178327. PMID 28053983.
- ^ Diao X, Deng P, Xie C, Li X, Zhong D, Zhang Y, Chen X. Metabolism and pharmacokinetics of 3-n-butylphthalide (NBP) in humans: the role of cytochrome P450s and alcohol dehydrogenase in biotransformation. Drug Metab Dispos 2013;41:430-444.
- ^ Diao X, Pang X, Xie C, Guo Z, Zhong D, Chen X. Bioactivation of 3-n-butylphthalide via sulfation of its major metabolite 3-hydroxy-NBP: mediated mainly by sulfotransferase 1A1. Drug Metab Dispos 2014;42:774-781.