Antiviral proteins are proteins that are induced by human or animal cells to interfere with viral replication. These proteins are isolated to inhibit the virus from replicating in a host's cells and stop it from spreading to other cells.[ citation needed] The Pokeweed antiviral protein and the Zinc-Finger antiviral protein are two major antiviral proteins that have undergone several tests for viruses, including HIV and influenza.[ citation needed]
Pokeweed antiviral protein
Pokeweed antiviral protein (PAP) is a ribosome inactivating protein that provides pokeweed plants protection against both viral and fungal infections. [1] It also protects other types of plants that have genetically engineered to express RAP that do not normally do so. [1] Recombinant PAP has also been proposed as a treatment of human diseases such as AIDS and cancer. [2] [3]
ZC3HAV1
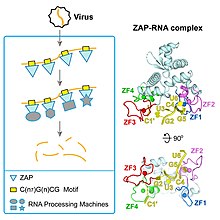
ZAP (Zinc finger Antiviral Protein) is encoded by the ZC3HAV1 gene [4] whose expression is induced by interferon and helps fight a number of viral infections including influenza. [5]
IFITM3
Interferon-induced transmembrane protein 3 ( IFITM3) inhibits the replication of number of enveloped RNA viruses including influenza A, HIV and the Ebola and Dengue viruses. [6] Consequently pharmacological induction of IFITM3 potentially could be used to treat a number of viral infections. [5]
Protein kinase R
Protein kinase R is interferon stimulated and activated either by double-stranded RNA (occurring as an intermediate in RNA viruses replication) or by other proteins. It is able to phosphorylate the eukaryotic translation initiation factor eIF2α thus inhibiting further cellular mRNA translation. [7]
References
- ^ a b Di R, Tumer NE (March 2015). "Pokeweed antiviral protein: its cytotoxicity mechanism and applications in plant disease resistance". Toxins. 7 (3): 755–72. doi: 10.3390/toxins7030755. PMC 4379523. PMID 25756953.
- ^ Rajamohan F, Engstrom CR, Denton TJ, Engen LA, Kourinov I, Uckun FM (July 1999). "High-level expression and purification of biologically active recombinant pokeweed antiviral protein". Protein Expression and Purification. 16 (2): 359–68. doi: 10.1006/prep.1999.1084. PMID 10419833.
- ^ Uckun FM, Rajamohan F, Pendergrass S, Ozer Z, Waurzyniak B, Mao C (March 2003). "Structure-based design and engineering of a nontoxic recombinant pokeweed antiviral protein with potent anti-human immunodeficiency virus activity". Antimicrobial Agents and Chemotherapy. 47 (3): 1052–61. doi: 10.1128/aac.47.3.1052-1061.2003. PMC 149289. PMID 12604541.
- ^ Gupte R, Liu Z, Kraus WL (January 2017). "PARPs and ADP-ribosylation: recent advances linking molecular functions to biological outcomes". Genes & Development. 31 (2): 101–126. doi: 10.1101/gad.291518.116. PMC 5322727. PMID 28202539.
- ^ a b Bedford JG, O'Keeffe M, Reading PC, Wakim LM (2019). "Rapid interferon independent expression of IFITM3 following T cell activation protects cells from influenza virus infection". PLOS ONE. 14 (1): e0210132. Bibcode: 2019PLoSO..1410132B. doi: 10.1371/journal.pone.0210132. PMC 6334895. PMID 30650117.
- ^ Wellington D, Laurenson-Schafer H, Abdel-Haq A, Dong T (February 2019). "IFITM3: How genetics influence influenza infection demographically". Biomedical Journal. 42 (1): 19–26. doi: 10.1016/j.bj.2019.01.004. PMC 6468115. PMID 30987701.
- ^ Fensterl, V.; Sen, G. C. (2009), "Interferons and viral infections", BioFactors, 35 (1): 14–20, doi: 10.1002/biof.6, PMID 19319841, S2CID 27209861