| |||
| |||
| Names | |||
|---|---|---|---|
|
IUPAC name
Ammonium ion
| |||
|
Systematic IUPAC name
Azanium
[1] | |||
| Identifiers | |||
3D model (
JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| MeSH | D000644 | ||
PubChem
CID
|
|||
| UNII | |||
CompTox Dashboard (
EPA)
|
|||
| |||
| |||
| Properties | |||
| [NH4+ | |||
| Molar mass | 18.039 g·mol−1 | ||
| Acidity (pKa) | 9.25 | ||
| Conjugate base | Ammonia | ||
| Structure | |||
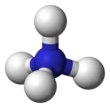
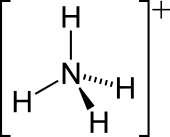
| Tetrahedral | |||
| Related compounds | |||
Other
cations
|
| ||
Related compounds
|
Ammonium radical •NH4 | ||
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |||
The ammonium cation is a positively charged polyatomic ion with the chemical formula NH+4 or [NH4+. It is formed by the protonation of ammonia (NH3). Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations ([NR4+), where one or more hydrogen atoms are replaced by organic or other groups (indicated by R). Not only is ammonium a source of nitrogen and a key metabolite for many living organisms, but it is an integral part of the global nitrogen cycle. [2] As such, the human impact in recent years could have an effect on the biological communities that depend on it.
Acid–base properties

The ammonium ion is generated when ammonia, a weak base, reacts with Brønsted acids ( proton donors):
- H+ + NH3 → [NH4+
The ammonium ion is mildly acidic, reacting with Brønsted bases to return to the uncharged ammonia molecule:
- [NH4+ + B− → HB + NH3
Thus, the treatment of concentrated solutions of ammonium salts with a strong base gives ammonia. When ammonia is dissolved in water, a tiny amount of it converts to ammonium ions:
- H2O + NH3 ⇌ OH− + [NH4+
The degree to which ammonia forms the ammonium ion depends on the pH of the solution. If the pH is low, the equilibrium shifts to the right: more ammonia molecules are converted into ammonium ions. If the pH is high (the concentration of hydrogen ions is low and hydroxide ions is high), the equilibrium shifts to the left: the hydroxide ion abstracts a proton from the ammonium ion, generating ammonia.
Formation of ammonium compounds can also occur in the vapor phase; for example, when ammonia vapor comes in contact with hydrogen chloride vapor, a white cloud of ammonium chloride forms, which eventually settles out as a solid in a thin white layer on surfaces.
Salts and characteristic reactions

Ammonium cation is found in a variety of salts such as ammonium carbonate, ammonium chloride, and ammonium nitrate. Most simple ammonium salts are very soluble in water. An exception is ammonium hexachloroplatinate, the formation of which was once used as a test for ammonium. The ammonium salts of nitrate and especially perchlorate are highly explosive, in these cases, ammonium is the reducing agent.
In an unusual process, ammonium ions form an amalgam. Such species are prepared by the addition of sodium amalgam to a solution of ammonium chloride. [3] This amalgam eventually decomposes to release ammonia and hydrogen. [4]
To find whether the ammonium ion is present in the salt, first, the salt is heated in presence of alkali hydroxide releasing a gas with a characteristic smell, which is ammonia.
- [NH4+ + OH− NH3 + H2O
To further confirm ammonia, it passed through a glass rod dipped in an HCl solution ( hydrochloric acid), creating white dense fumes of ammonium chloride.
- NH3(g) + HCl(aq) → [NH4]Cl(s)
Ammonia, when passed through CuSO4 ( copper(II) sulfate) solution, changes its color from blue to deep blue, forming Schweizer's reagent.
- CuSO4(aq) + 4 NH3(aq) + 4 H2O → [Cu(NH3)4(H2O)2](OH)2(aq) + H2SO4(aq)
Ammonia or ammonium ion when added to Nessler's reagent gives a brown color precipitate known as the iodide of Million's base in basic medium.
Ammonium ion when added to chloroplatinic acid gives a yellow precipitate of ammonium hexachloroplatinate(IV).
- H2[PtCl6](aq) + [NH4+(aq) → [NH42[PtCl6](s) + 2 H+
Ammonium ion when added to sodium cobaltinitrite gives a yellow precipitate of ammonium cobaltinitrite.
- Na3[Co(NO2)6](aq) + 3 [NH4+(aq) → [NH43[Co(NO2)6](s) + 3 Na+(aq)
Ammonium ion gives a white precipitate of ammonium bitartrate when added to potassium bitartrate.
- KC4H5O6(aq) + [NH4+(aq) → [NH4]C4H5O6(s) + K+(aq)
Structure and bonding
This section needs additional citations for
verification. (March 2022) |
The lone electron pair on the nitrogen atom (N) in ammonia, represented as a line above the N, forms a coordinate bond with a proton (H+). After that, all four N−H bonds are equivalent, being polar covalent bonds. The ion has a tetrahedral structure and is isoelectronic with methane and the borohydride anion. In terms of size, the ammonium cation (rionic = 175 pm)[ citation needed] resembles the caesium cation (rionic = 183 pm).[ citation needed]
Organic ions
The hydrogen atoms in the ammonium ion can be substituted with an alkyl group or some other organic group to form a substituted ammonium ion ( IUPAC nomenclature: aminium ion). Depending on the number of organic groups, the ammonium cation is called a primary, secondary, tertiary, or quaternary. Except the quaternary ammonium cations, the organic ammonium cations are weak acids.
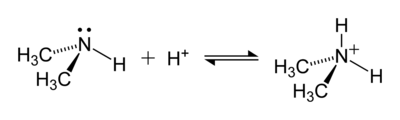
An example of a reaction forming an ammonium ion is that between dimethylamine, (CH3)2NH, and an acid to give the dimethylammonium cation, [(CH3)2NH2+:
Quaternary ammonium cations have four organic groups attached to the nitrogen atom, they lack a hydrogen atom bonded to the nitrogen atom. These cations, such as the tetra-n-butylammonium cation, are sometimes used to replace sodium or potassium ions to increase the solubility of the associated anion in organic solvents. Primary, secondary, and tertiary ammonium salts serve the same function but are less lipophilic. They are also used as phase-transfer catalysts and surfactants.
An unusual class of organic ammonium salts is derivatives of amine radical cations, [•NR3+ such as tris(4-bromophenyl)ammoniumyl hexachloroantimonate.
Biology

Because nitrogen often limits net primary production due to its use in enzymes that mediate the biochemical reactions that are necessary for life, ammonium is utilized by some microbes and plants. [5] For example, energy is released by the oxidation of ammonium in a process known as nitrification, which produces nitrate and nitrite. [6] This process is a form of autotrophy that is common amongst Nitrosomonas, Nitrobacter, Nitrosolobus, and Nitrosospira, amongst others. [6]
The amount of ammonium in soil that is available for nitrification by microbes varies depending on environmental conditions. [7] [8] For example, ammonium is deposited as a waste product from some animals, although it is converted into urea in mammals, sharks, and amphibians, and into uric acid in birds, reptiles, and terrestrial snails. [9] Its availability in soils is also influenced by mineralization, which makes more ammonium available from organic nitrogen sources, and immobilization, which sequesters ammonium into organic nitrogen sources, both of which are mitigated by biological factors. [6]
Conversely, nitrate and nitrite can be reduced to ammonium as a way for living organisms to access nitrogen for growth in a process known as assimilatory nitrate reduction. [10] Once assimilated, it can be incorporated into proteins and DNA. [11]
Ammonium can accumulate in soils where nitrification is slow or inhibited, which is common in hypoxic soils. [12] For example, ammonium mobilization is one of the key factors for the symbiotic association between plants and fungi, called mycorrhizae. [13] However, plants that consistently utilize ammonium as a nitrogen source often must invest into more extensive root systems due to ammonium's limited mobility in soils compared to other nitrogen sources. [14] [15]
Human Impact
Ammonium deposition from the atmosphere has increased in recent years due to volatilization from livestock waste and increased fertilizer use. [16] Because net primary production is often limited by nitrogen, increased ammonium levels could impact the biological communities that rely on it. For example, increasing nitrogen content has been shown to increase plant growth, but aggravate soil phosphorus levels, which can impact microbial communities. [17]
Metal
The ammonium cation has very similar properties to the heavier alkali metal cations and is often considered a close equivalent. [18] [19] [20] Ammonium is expected to behave as a metal ([NH4+ ions in a sea of electrons) at very high pressures, such as inside giant gas planets such as Uranus and Neptune. [19] [20]
Under normal conditions, ammonium does not exist as a pure metal but does as an amalgam (alloy with mercury). [21]
See also
- Onium compounds
- Fluoronium, ([H2F]+ and substituted derivatives)
- Oxonium ([R3O]+, where R is typically hydrogen or organyl)
- Hydronium ([H3O]+, the simplest oxonium ion)
- Quaternary ammonium cation ([NR4+, where R is organyl)
- Tetrafluoroammonium ([NF4+)
- Hydrazinium ([H2N−NH3+ and substituted derivatives)
- Hydrazinediium ([H3N−NH32+ and substituted derivatives)
- Iminium ([H2C=NH2+ and substituted derivatives)
- Diazonium ([H−N≡N]+ and substituted derivatives)
- Diazynediium ([H−N≡N−H]2+ and substituted derivatives)
- Aminodiazonium ([H2N=N=N]+ ⇌ [H2N−N≡N]+ and substituted derivatives)
- Hydroxylammonium ([HO−NH3+ and substituted derivatives)
- Ammonium transporter
- f-ratio
- Nitrification
- The Magnificent Possession (Isaac Asimov short story)
- Ammonium hydroxide
References
- ^ International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC– IUPAC. ISBN 0-85404-438-8. pp. 71,105,314. Electronic version.
- ^ Schlesinger, William H.; Bernhardt, Emily S. (2020-01-01), Schlesinger, William H.; Bernhardt, Emily S. (eds.), "Chapter 12 - The Global Cycles of Nitrogen, Phosphorus and Potassium", Biogeochemistry (Fourth Edition), Academic Press, pp. 483–508, doi: 10.1016/b978-0-12-814608-8.00012-8, ISBN 978-0-12-814608-8, retrieved 2024-03-08
- ^ "Pseudo-binary compounds". Archived from the original on 2020-07-27. Retrieved 2007-10-12.
- ^ "Ammonium Salts". VIAS Encyclopedia.
- ^ Schlesinger, William H.; Bernhardt, Emily S. (2020-01-01), Schlesinger, William H.; Bernhardt, Emily S. (eds.), "Chapter 12 - The Global Cycles of Nitrogen, Phosphorus and Potassium", Biogeochemistry (Fourth Edition), Academic Press, pp. 483–508, doi: 10.1016/b978-0-12-814608-8.00012-8, ISBN 978-0-12-814608-8, retrieved 2024-03-08
- ^ a b c Rosswall, T. (1982). "Microbiological regulation of the biogeochemical nitrogen cycle / Regulación microbiana del ciclo bíogeoquímico del nitrógeno". Plant and Soil. 67 (1/3): 15–34. doi: 10.1007/BF02182752. ISSN 0032-079X. JSTOR 42934020.
- ^ Barsdate, Robert J.; Alexander, Vera (January 1975). "The Nitrogen Balance of Arctic Tundra: Pathways, Rates, and Environmental Implications". Journal of Environmental Quality. 4 (1): 111–117. Bibcode: 1975JEnvQ...4..111B. doi: 10.2134/jeq1975.00472425000400010025x. ISSN 0047-2425.
- ^ Nadelhoffer, Knute J.; Aber, John D.; Melillo, Jerry M. (1984-10-01). "Seasonal patterns of ammonium and nitrate uptake in nine temperate forest ecosystems". Plant and Soil. 80 (3): 321–335. Bibcode: 1984PlSoi..80..321N. doi: 10.1007/BF02140039. ISSN 1573-5036.
- ^ Campbell, Neil A.; Reece, Jane B. (2002). Biology. Internet Archive. San Francisco : Benjamin Cummings. ISBN 978-0-8053-6624-2.
- ^ Tiedje, J. M.; Sørensen, J.; Chang, Y.-Y. L. (1981). "Assimilatory and Dissimilatory Nitrate Reduction: Perspectives and Methodology for Simultaneous Measurement of Several Nitrogen Cycle Processes". Ecological Bulletins (33): 331–342. ISSN 0346-6868. JSTOR 45128674.
- ^ Llácer, José L; Fita, Ignacio; Rubio, Vicente (2008-12-01). "Arginine and nitrogen storage". Current Opinion in Structural Biology. Catalysis and regulation / Proteins. 18 (6): 673–681. doi: 10.1016/j.sbi.2008.11.002. ISSN 0959-440X. PMID 19013524.
- ^ Wang, Lixin; Macko, Stephen A. (March 2011). "Constrained preferences in nitrogen uptake across plant species and environments". Plant, Cell & Environment. 34 (3): 525–534. doi: 10.1111/j.1365-3040.2010.02260.x. ISSN 0140-7791. PMID 21118424.
- ^ Hodge, Angela; Storer, Kate (2015-01-01). "Arbuscular mycorrhiza and nitrogen: implications for individual plants through to ecosystems". Plant and Soil. 386 (1): 1–19. Bibcode: 2015PlSoi.386....1H. doi: 10.1007/s11104-014-2162-1. ISSN 1573-5036.
- ^ Raven, John A.; Linda, Bernd Wollenweber; Handley, L. (May 1992). "Ammonia and ammonium fluxes between photolithotrophs and the environment in relation to the global nitrogen cycle". New Phytologist. 121 (1): 5–18. doi: 10.1111/j.1469-8137.1992.tb01087.x. ISSN 0028-646X.
- ^ Bloom, A. J.; Jackson, L. E.; Smart, D. R. (March 1993). "Root growth as a function of ammonium and nitrate in the root zone". Plant, Cell & Environment. 16 (2): 199–206. doi: 10.1111/j.1365-3040.1993.tb00861.x. ISSN 0140-7791.
- ^ Ackerman, Daniel; Millet, Dylan B.; Chen, Xin (January 2019). "Global Estimates of Inorganic Nitrogen Deposition Across Four Decades". Global Biogeochemical Cycles. 33 (1): 100–107. Bibcode: 2019GBioC..33..100A. doi: 10.1029/2018GB005990. ISSN 0886-6236.
- ^ Dong, Junfu; Cui, Xiaoyong; Niu, Haishan; Zhang, Jing; Zhu, Chuanlu; Li, Linfeng; Pang, Zhe; Wang, Shiping (2022-06-20). "Effects of Nitrogen Addition on Plant Properties and Microbiomes Under High Phosphorus Addition Level in the Alpine Steppe". Frontiers in Plant Science. 13. doi: 10.3389/fpls.2022.894365. ISSN 1664-462X. PMC 9251499. PMID 35795351.
- ^ Holleman, Arnold Frederik; Wiberg, Egon (2001), Wiberg, Nils (ed.), Inorganic Chemistry, translated by Eagleson, Mary; Brewer, William, San Diego/Berlin: Academic Press/De Gruyter, ISBN 0-12-352651-5
- ^ a b Stevenson, D. J. (November 20, 1975). "Does metallic ammonium exist?". Nature. 258 (5532): 222–223. Bibcode: 1975Natur.258..222S. doi: 10.1038/258222a0. S2CID 4199721.
- ^ a b Bernal, M. J. M.; Massey, H. S. W. (February 3, 1954). "Metallic Ammonium". Monthly Notices of the Royal Astronomical Society. 114 (2): 172–179. Bibcode: 1954MNRAS.114..172B. doi: 10.1093/mnras/114.2.172.
- ^ Reedy, J.H. (October 1, 1929). "Lecture demonstration of ammonium amalgam". Journal of Chemical Education. 6 (10): 1767. Bibcode: 1929JChEd...6.1767R. doi: 10.1021/ed006p1767.