 | |
| Clinical data | |
|---|---|
| Trade names | Nesina, Vipidia Kazano, Vipidomet (with metformin) Oseni, Incresync (with pioglitazone) |
| Other names | SYR-322 |
| AHFS/ Drugs.com | Monograph |
| MedlinePlus | a613026 |
| License data |
|
|
Pregnancy category |
|
|
Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% |
| Protein binding | 20% |
| Metabolism | Limited, liver ( CYP2D6- and 3A4-mediated) |
| Elimination half-life | 12–21 hours |
| Excretion | Kidney (major) [1] and fecal (minor) |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| ECHA InfoCard | 100.256.501 |
| Chemical and physical data | |
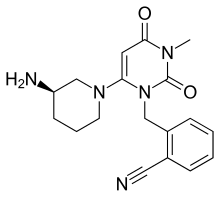
| Formula | C18H21N5O2 |
| Molar mass | 339.399 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
| | |
Alogliptin, sold under the brand names Nesina and Vipidia, [2] [3] is an oral anti-diabetic drug in the DPP-4 inhibitor (gliptin) class. [4] Like other members of the gliptin class, it causes little or no weight gain, exhibits relatively little risk of hypoglycemia, and has relatively modest glucose-lowering activity. [1] Alogliptin and other gliptins are commonly used in combination with metformin in people whose diabetes cannot adequately be controlled with metformin alone. [1]
In April 2016, the U.S. Food and Drug Administration (FDA) added a warning about increased risk of heart failure. [5] It was developed by Syrrx, a company which was acquired by Takeda Pharmaceutical Company in 2005. [6] In 2020, it was the 295th most commonly prescribed medication in the United States, with more than 1 million prescriptions. [7] [8]
Medical uses
Alogliptin is a dipeptidyl peptidase-4 inhibitor (DDP-4) that decreases blood sugar levels similar to other DPP-4 inhibitors. [9]
Side effects
Adverse events include hypoglycemia, [10] [11] [12] pruritis (itching), [3] nasopharyngitis, headache, and upper respiratory tract infection. [13] It may also cause joint pain that can be severe and disabling. [14] Like other DDP-4 inhibitors, alogliptin is weight-neutral. [1]
A 2014 letter to the editor claimed alogliptin is not associated with increased risk of cardiovascular events. [15][ better source needed] In April 2016, the U.S. Food and Drug Administration (FDA) added a warning about increased risk of heart failure. [5]
Market access

In December 2007, Takeda submitted a New Drug Application (NDA) for alogliptin to the United States Food and Drug Administration (FDA), [16] after positive results from Phase III clinical trials. [2] In September 2008, the company also filed for approval in Japan, [17] winning approval in April 2010. [16] The company also filed a Marketing Authorization Application elsewhere outside the United States, which was withdrawn in June 2009 needing more data. [17] The first NDA failed to gain approval and was followed by a pair of NDAs (one for alogliptin and a second for a combination of alogliptin and pioglitazone) in July 2011. [16] In 2012, Takeda received a negative response from the FDA on both of these NDAs, citing a need for additional data. [16]
In 2013, the FDA approved the drug in three formulations: as a stand-alone with the brand-name Nesina, [13] combined with metformin using the name Kazano, [18] and when combined with pioglitazone as Oseni. [19]
References
- ^ a b c d "www.aace.com" (PDF). Archived from the original (PDF) on 2018-11-01.
- ^ a b "Takeda Submits New Drug Application for Alogliptin (SYR-322) in the U.S." (Press release). Takeda Pharmaceutical Company. January 3, 2008. Retrieved March 11, 2021.
- ^ a b "Vipidia" (PDF). European Medicines Agency. Archived from the original (PDF) on 1 November 2018. Retrieved 31 March 2024.
- ^ Feng J, Zhang Z, Wallace MB, Stafford JA, Kaldor SW, Kassel DB, et al. (May 2007). "Discovery of alogliptin: a potent, selective, bioavailable, and efficacious inhibitor of dipeptidyl peptidase IV". Journal of Medicinal Chemistry. 50 (10): 2297–2300. doi: 10.1021/jm070104l. PMID 17441705.
- ^ a b "FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin". U.S. Food and Drug Administration (FDA). Retrieved 16 March 2018.
- ^ "The San Diego Union-Tribune - San Diego, California & National News".
- ^ "The Top 300 of 2020". ClinCalc. Retrieved 7 October 2022.
- ^ "Alogliptin - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.
- ^ Saisho Y (2015). "Alogliptin benzoate for management of type 2 diabetes". Vascular Health and Risk Management. 11: 229–243. doi: 10.2147/VHRM.S68564. PMC 4401208. PMID 25914541.
- ^ Seino Y, Fujita T, Hiroi S, Hirayama M, Kaku K (September 2011). "Efficacy and safety of alogliptin in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, dose-ranging comparison with placebo, followed by a long-term extension study". Current Medical Research and Opinion. 27 (9): 1781–1792. doi: 10.1185/03007995.2011.599371. PMID 21806314. S2CID 24082863.
- ^ Kutoh E, Ukai Y (June 2012). "Alogliptin as an initial therapy in patients with newly diagnosed, drug naïve type 2 diabetes: a randomized, control trial". Endocrine. 41 (3) (published January 17, 2012): 435–441. doi: 10.1007/s12020-012-9596-0. PMID 22249941. S2CID 45948727.
- ^ Bosi E, Ellis GC, Wilson CA, Fleck PR (December 2011). "Alogliptin as a third oral antidiabetic drug in patients with type 2 diabetes and inadequate glycaemic control on metformin and pioglitazone: a 52-week, randomized, double-blind, active-controlled, parallel-group study". Diabetes, Obesity & Metabolism. 13 (12) (published October 27, 2011): 1088–1096. doi: 10.1111/j.1463-1326.2011.01463.x. PMID 21733058. S2CID 1092260.
- ^ a b "Highlights of Prescribing Information: Nesina" (PDF). US Food and Drug Administration. Retrieved 31 March 2024.
- ^ "DPP-4 Inhibitors for Type 2 Diabetes: Drug Safety Communication - May Cause Severe Joint Pain". U.S. Food and Drug Administration (FDA). 2015-08-28. Retrieved 1 September 2015.
- ^ White WB, Zannad F (January 2014). "Saxagliptin, alogliptin, and cardiovascular outcomes". The New England Journal of Medicine. 370 (5): 484. doi: 10.1056/NEJMc1313880. PMID 24482824.
- ^ a b c d Grogan K (April 26, 2012), "FDA wants yet more data on Takeda diabetes drug alogliptin", PharmaTimes, PharmaTimes, PharmaTimes online, retrieved April 26, 2012
- ^ a b "GEN News Highlights: Takeda Pulls MAA for Type 2 Diabetes Therapy". Genetic Engineering & Biotechnology News. June 4, 2009.
- ^ "Highlights of Prescribing Information: Kazano" (PDF). US Food and Drug Administration. Retrieved 31 March 2024.
- ^ "Highlights of Prescribing Information: Oseni" (PDF). US Food and Drug Administration. Retrieved 31 March 2024.
External links
-
 Media related to
Alogliptin at Wikimedia Commons
Media related to
Alogliptin at Wikimedia Commons - "Alogliptin". Drug Information Portal. U.S. National Library of Medicine.