| |
| Identifiers | |
|---|---|
3D model (
JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.208.660 |
PubChem
CID
|
|
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
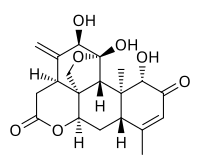
| C20H24O7 | |
| Molar mass | 376.405 g·mol−1 |
| Density | 1.47 g/cm3 |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Ailanthone is an allelopathic chemical that is produced by the Ailanthus altissima tree which inhibits the growth of other plants. [1]
References
- ^ Heisy, Rod M. (February 1996). "Identification of an Allelopathic Compound from Ailanthus altissima (Simaroubaceae) and Characterization of its Herbicidal Activity". American Journal of Botany. 83 (2): 192–200. doi: 10.2307/2445938. JSTOR 2445938.