Advanced glycation end products (AGEs) are proteins or lipids that become glycated as a result of exposure to sugars. [1] They are a bio-marker implicated in aging and the development, or worsening, of many degenerative diseases, such as diabetes, atherosclerosis, chronic kidney disease, and Alzheimer's disease. [2]
Dietary sources
Animal-derived foods that are high in fat and protein are generally AGE-rich and are prone to further AGE formation during cooking. [3] However, only low molecular weight AGEs are absorbed through diet, and vegetarians have been found to have higher concentrations of overall AGEs compared to non-vegetarians. [4] Therefore, it is unclear whether dietary AGEs contribute to disease and aging, or whether only endogenous AGEs (those produced in the body) matter. [5] This does not free diet from potentially negatively influencing AGE, but potentially implies that dietary AGE may deserve less attention than other aspects of diet that lead to elevated blood sugar levels and formation of AGEs. [4] [5]
Effects

AGEs affect nearly every type of cell and molecule in the body and are thought to be one factor in aging [6] and some age-related chronic diseases. [7] [8] [9] They are also believed to play a causative role in the vascular complications of diabetes mellitus. [10]
AGEs arise under certain pathologic conditions, such as oxidative stress due to hyperglycemia in patients with diabetes. [11] AGEs play a role as proinflammatory mediators in gestational diabetes as well. [12]
In the context of cardiovascular disease, AGEs can induce crosslinking of collagen, which can cause vascular stiffening and entrapment of low-density lipoprotein particles (LDL) in the artery walls. AGEs can also cause glycation of LDL which can promote its oxidation. [13] Oxidized LDL is one of the major factors in the development of atherosclerosis. [14] Finally, AGEs can bind to RAGE (receptor for advanced glycation end products) and cause oxidative stress as well as activation of inflammatory pathways in vascular endothelial cells. [13] [14]
In other diseases
AGEs have been implicated in Alzheimer's Disease, [15] cardiovascular disease, [16] and stroke. [17] The mechanism by which AGEs induce damage is through a process called cross-linking that causes intracellular damage and apoptosis. [18] They form photosensitizers in the crystalline lens, [19] which has implications for cataract development. [20] Reduced muscle function is also associated with AGEs. [21]
Pathology
AGEs have a range of pathological effects, such as: [22] [23]
- Increased vascular permeability.
- Increased arterial stiffness
- Inhibition of vascular dilation by interfering with nitric oxide.
- Oxidizing LDL.
- Binding cells—including macrophage, endothelial, and mesangial—to induce the secretion of a variety of cytokines.
- Enhanced oxidative stress.
- Hemoglobin-AGE levels are elevated in diabetic individuals [24] and other AGE proteins have been shown in experimental models to accumulate with time, increasing from 5-50 fold over periods of 5–20 weeks in the retina, lens and renal cortex of diabetic rats. The inhibition of AGE formation reduced the extent of nephropathy in diabetic rats. [25] Therefore, substances that inhibit AGE formation may limit the progression of disease and may offer new tools for therapeutic interventions in the therapy of AGE-mediated disease. [26] [27]
- AGEs have specific cellular receptors; the best-characterized are those called RAGE. The activation of cellular RAGE on endothelium, mononuclear phagocytes, and lymphocytes triggers the generation of free radicals and the expression of inflammatory gene mediators. [28] Such increases in oxidative stress lead to the activation of the transcription factor NF-κB and promote the expression of NF-κB regulated genes that have been associated with atherosclerosis. [26]
Reactivity
Proteins are usually glycated through their lysine residues. [29] In humans, histones in the cell nucleus are richest in lysine, and therefore form the glycated protein N(6)-Carboxymethyllysine (CML). [29]
A receptor nicknamed RAGE, from receptor for advanced glycation end products, is found on many cells, including endothelial cells, smooth muscle, cells of the immune system [ which?] from tissue such as lung, liver, and kidney.[ clarification needed][ which?] This receptor, when binding AGEs, contributes to age- and diabetes-related chronic inflammatory diseases such as atherosclerosis, asthma, arthritis, myocardial infarction, nephropathy, retinopathy, periodontitis and neuropathy. [30] The pathogenesis of this process hypothesized to activation of the nuclear factor kappa B ( NF-κB) following AGE binding. [31] NF-κB controls several genes which are involved in inflammation. [32] AGEs can be detected and quantified using bioanalytical and immunological methods. [33]
Clearance
In clearance, or the rate at which a substance is removed or cleared from the body, it has been found that the cellular proteolysis of AGEs—the breakdown of proteins—produces AGE peptides and "AGE free adducts" (AGE adducts bound to single amino acids). These latter, after being released into the plasma, can be excreted in the urine. [34]
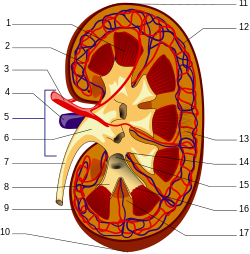
Nevertheless, the resistance of extracellular matrix proteins to proteolysis renders their advanced glycation end products less conducive to being eliminated. [34] While the AGE free adducts are released directly into the urine, AGE peptides are endocytosed by the epithelial cells of the proximal tubule and then degraded by the endolysosomal system to produce AGE amino acids. It is thought that these acids are then returned to the kidney's inside space, or lumen, for excretion. [22] AGE free adducts are the major form through which AGEs are excreted in urine, with AGE-peptides occurring to a lesser extent [22] but accumulating in the plasma of patients with chronic kidney failure. [34]
Larger, extracellularly derived AGE proteins cannot pass through the basement membrane of the renal corpuscle and must first be degraded into AGE peptides and AGE free adducts. Peripheral macrophage [22] as well as liver sinusoidal endothelial cells and Kupffer cells [35] have been implicated in this process, although the real-life involvement of the liver has been disputed. [36]

Large AGE proteins unable to enter the Bowman's capsule are capable of binding to receptors on endothelial and mesangial cells and to the mesangial matrix. [22] Activation of RAGE induces production of a variety of cytokines, including TNFβ, which mediates an inhibition of metalloproteinase and increases production of mesangial matrix, leading to glomerulosclerosis [23] and decreasing kidney function in patients with unusually high AGE levels.
Although the only form suitable for urinary excretion, the breakdown products of AGE—that is, peptides and free adducts—are more aggressive than the AGE proteins from which they are derived, and they can perpetuate related pathology in diabetic patients, even after hyperglycemia has been brought under control. [22]
Some AGEs have an innate catalytic oxidative capacity, while activation of NAD(P)H oxidase through activation of RAGE and damage to mitochondrial proteins leading to mitochondrial dysfunction can also induce oxidative stress. A 2007 in vitro study found that AGEs could significantly increase expression of TGF-β1, CTGF, Fn mRNA in NRK-49F cells through enhancement of oxidative stress, and suggested that inhibition of oxidative stress might underlie the effect of ginkgo biloba extract in diabetic nephropathy. The authors suggested that antioxidant therapy might help prevent the accumulation of AGEs and induced damage. [23] In the end, effective clearance is necessary, and those suffering AGE increases because of kidney dysfunction might require a kidney transplant. [22]
In diabetics who have an increased production of an AGE, kidney damage reduces the subsequent urinary removal of AGEs, forming a positive feedback loop that increases the rate of damage. In a 1997 study, diabetic and healthy subjects were given a single meal of egg white (56 g protein), cooked with or without 100 g of fructose; there was a greater than 200-fold increase in AGE immunoreactivity from the meal with fructose. [37]
Potential therapy

AGEs are the subject of ongoing research. There are three therapeutic approaches: preventing the formation of AGEs, breaking crosslinks after they are formed and preventing their negative effects.
Compounds that have been found to inhibit AGE formation in the laboratory include Vitamin C, Agmatine, benfotiamine, pyridoxamine, alpha-lipoic acid, [38] [39] taurine, [40] pimagedine, [41] aspirin, [42] [43] carnosine, [44] metformin, [45] pioglitazone, [45] and pentoxifylline. [45] Activation of the TRPA-1 receptor by lipoic acid or podocarpic acid has been shown to reduce the levels of AGES by enhancing the detoxification of methylglyoxal, a major precursor of several AGEs. [38]
Studies in rats and mice have found that natural phenols such as resveratrol and curcumin can prevent the negative effects of the AGEs. [46] [47]
Compounds that are thought to break some existing AGE crosslinks include Alagebrium (and related ALT-462, ALT-486, and ALT-946) [48] and N-phenacyl thiazolium bromide. [49] One in vitro study shows that rosmarinic acid out performs the AGE breaking potential of ALT-711. [50]

There is, however, no agent known that can break down the most common AGE, glucosepane, which appears 10 to 1,000 times more common in human tissue than any other cross-linking AGE. [51] [52]
Some chemicals, on the other hand, like aminoguanidine, might limit the formation of AGEs by reacting with 3-deoxyglucosone. [30]
See also
References
- ^ Goldin, Alison; Beckman, Joshua A.; Schmidt, Ann Marie; Creager, Mark A. (2006). "Advanced Glycation End Products Sparking the Development of Diabetic Vascular Injury". Circulation. 114 (6): 597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. PMID 16894049.
- ^ Vistoli, G; De Maddis, D; Cipak, A; Zarkovic, N; Carini, M; Aldini, G (Aug 2013). "Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation" (PDF). Free Radic Res. 47: Suppl 1:3–27. doi: 10.3109/10715762.2013.815348. PMID 23767955. S2CID 207517855.
- ^ Uribarri, Jaime; Woodruff, Sandra; Goodman, Susan; Cai, Weijing; Chen, Xue; Pyzik, Renata; Yong, Angie; Striker, Gary E.; Vlassara, Helen (June 2010). "Advanced Glycation End Products in Foods and a Practical Guide to Their Reduction in the Diet". Journal of the American Dietetic Association. 110 (6): 911–916.e12. doi: 10.1016/j.jada.2010.03.018. PMC 3704564. PMID 20497781.
- ^ a b Poulsen, Malene W.; Hedegaard, Rikke V.; Andersen, Jeanette M.; de Courten, Barbora; Bügel, Susanne; Nielsen, John; Skibsted, Leif H.; Dragsted, Lars O. (October 2013). "Advanced glycation endproducts in food and their effects on health". Food and Chemical Toxicology. 60: 10–37. doi: 10.1016/j.fct.2013.06.052. PMID 23867544.
- ^ a b Luevano-Contreras, Claudia; Chapman-Novakofski, Karen (13 December 2010). "Dietary Advanced Glycation End Products and Aging". Nutrients. 2 (12): 1247–1265. doi: 10.3390/nu2121247. PMC 3257625. PMID 22254007.
- ^ Chaudhuri, Jyotiska; Bains, Yasmin; Guha, Sanjib; Kahn, Arnold; Hall, David; Bose, Neelanjan; Gugliucci, Alejandro; Kapahi, Pankaj (4 September 2018). "The role of advanced glycation end products in aging and metabolic diseases: bridging association and causality". Cell Metabolism. 28 (3): 337–352. doi: 10.1016/j.cmet.2018.08.014. PMC 6355252. PMID 30184484.
- ^ Glenn, J.; Stitt, A. (2009). "The role of advanced glycation end products in retinal ageing and disease". Biochimica et Biophysica Acta (BBA) - General Subjects. 1790 (10): 1109–1116. doi: 10.1016/j.bbagen.2009.04.016. PMID 19409449.
- ^ Semba, R. D.; Ferrucci, L.; Sun, K.; Beck, J.; Dalal, M.; Varadhan, R.; Walston, J.; Guralnik, J. M.; Fried, L. P. (2009). "Advanced glycation end products and their circulating receptors predict cardiovascular disease mortality in older community-dwelling women". Aging Clinical and Experimental Research. 21 (2): 182–190. doi: 10.1007/BF03325227. PMC 2684987. PMID 19448391.
- ^ Semba, R.; Najjar, S.; Sun, K.; Lakatta, E.; Ferrucci, L. (2009). "Serum carboxymethyl-lysine, an advanced glycation end product, is associated with increased aortic pulse wave velocity in adults". American Journal of Hypertension. 22 (1): 74–79. doi: 10.1038/ajh.2008.320. PMC 2637811. PMID 19023277.
- ^ Yan, S. F.; D'Agati, V.; Schmidt, A. M.; Ramasamy, R. (2007). "Receptor for Advanced Glycation Endproducts (RAGE): a formidable force in the pathogenesis of the cardiovascular complications of diabetes & aging". Current Molecular Medicine. 7 (8): 699–710. doi: 10.2174/156652407783220732. PMID 18331228.
- ^ Brownlee, M (June 2005). "The pathobiology of diabetic complications: a unifying mechanism". Diabetes. 54 (6): 1615–25. doi: 10.2337/diabetes.54.6.1615. PMID 15919781.
- ^ Pertyńska-Marczewska, Magdalena; Głowacka, Ewa; Sobczak, Małgorzata; Cypryk, Katarzyna; Wilczyński, Jan (11 January 2009). "Glycation Endproducts, Soluble Receptor for Advanced Glycation Endproducts and Cytokines in Diabetic and Non-diabetic Pregnancies". American Journal of Reproductive Immunology. 61 (2): 175–182. doi: 10.1111/j.1600-0897.2008.00679.x. PMID 19143681. S2CID 3186554.
- ^ a b Prasad, Anand; Bekker, Peter; Tsimikas, Sotirios (2012). "Advanced Glycation End Products and Diabetic Cardiovascular Disease". Cardiology in Review. 20 (4): 177–183. doi: 10.1097/CRD.0b013e318244e57c. PMID 22314141. S2CID 8471652.
- ^ a b Di Marco, Elyse; Gray, Stephen P.; Jandeleit-Dahm, Karin (2013). "Diabetes Alters Activation and Repression of Pro- and Anti-Inflammatory Signaling Pathways in the Vasculature". Frontiers in Endocrinology. 4: 68. doi: 10.3389/fendo.2013.00068. PMC 3672854. PMID 23761786.
- ^ Srikanth, Velandai; Maczurek, Annette; Phan, Thanh; Steele, Megan; Westcott, Bernadette; Juskiw, Damian; Münch, Gerald (May 2011). "Advanced glycation endproducts and their receptor RAGE in Alzheimer's disease". Neurobiology of Aging. 32 (5): 763–777. doi: 10.1016/j.neurobiolaging.2009.04.016. PMID 19464758. S2CID 207158367.
- ^ Simm, A.; Wagner, J.; Gursinsky, T.; Nass, N.; Friedrich, I.; Schinzel, R.; Czeslik, E.; Silber, R.E.; Scheubel, R.J. (July 2007). "Advanced glycation endproducts: A biomarker for age as an outcome predictor after cardiac surgery?". Experimental Gerontology. 42 (7): 668–675. doi: 10.1016/j.exger.2007.03.006. PMID 17482402. S2CID 30264495.
- ^ Zimmerman, G A; Meistrell, M; Bloom, O; Cockroft, K M; Bianchi, M; Risucci, D; Broome, J; Farmer, P; Cerami, A; Vlassara, H (25 April 1995). "Neurotoxicity of advanced glycation endproducts during focal stroke and neuroprotective effects of aminoguanidine". Proceedings of the National Academy of Sciences of the United States of America. 92 (9): 3744–3748. Bibcode: 1995PNAS...92.3744Z. doi: 10.1073/pnas.92.9.3744. PMC 42038. PMID 7731977.
- ^ Shaikh, Shamim; Nicholson, Louise F.B. (July 2008). "Advanced glycation end products induce in vitro cross‐linking of α‐synuclein and accelerate the process of intracellular inclusion body formation". Journal of Neuroscience Research. 86 (9): 2071–2082. doi: 10.1002/jnr.21644. PMID 18335520. S2CID 37510479.
- ^ Fuentealba, Denis; Friguet, Bertrand; Silva, Eduardo (January 2009). "Advanced Glycation Endproducts Induce Photocrosslinking and Oxidation of Bovine Lens Proteins Through Type-I Mechanism". Photochemistry and Photobiology. 85 (1): 185–194. doi: 10.1111/j.1751-1097.2008.00415.x. PMID 18673320.
- ^ Gul, Anjuman; Rahman, M. Ataur; Hasnain, Syed Nazrul (6 February 2009). "Role of fructose concentration on cataractogenesis in senile diabetic and non-diabetic patients". Graefe's Archive for Clinical and Experimental Ophthalmology. 247 (6): 809–814. doi: 10.1007/s00417-008-1027-9. PMID 19198870. S2CID 9260375.
- ^ Haus, Jacob M.; Carrithers, John A.; Trappe, Scott W.; Trappe, Todd A. (December 2007). "Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle". Journal of Applied Physiology. 103 (6): 2068–2076. doi: 10.1152/japplphysiol.00670.2007. PMID 17901242.
- ^ a b c d e f g Gugliucci A, Bendayan M (1996). "Renal fate of circulating advanced glycated end products (AGE): evidence for reabsorption and catabolism of AGE peptides by renal proximal tubular cells". Diabetologia. 39 (2): 149–60. doi: 10.1007/BF00403957. PMID 8635666.
- ^ a b c Yan, Hai-dong; Li, Xue-zhu; Xie, Jun-mei; Li, Man (May 2007). "Effects of advanced glycation end products on renal fibrosis and oxidative stress in cultured NRK-49F cells". Chinese Medical Journal. 120 (9): 787–793. doi: 10.1097/00029330-200705010-00010. PMID 17531120.
- ^ Kostolanská J, Jakus V, Barák L (May 2009). "HbA1c and serum levels of advanced glycation and oxidation protein products in poorly and well controlled children and adolescents with type 1 diabetes mellitus". Journal of Pediatric Endocrinology & Metabolism. 22 (5): 433–42. doi: 10.1515/JPEM.2009.22.5.433. PMID 19618662. S2CID 23150519.
- ^ Ninomiya, T.; et al. (2001). "A novel AGE production inhibitor, prevents progression of diabetic nephropathy in STZ-induced rats". Diabetes. 50 Suppl. (2): A178–179.
- ^ a b Bierhaus A, Hofmann MA, Ziegler R, Nawroth PP (March 1998). "AGEs and their interaction with AGE-receptors in vascular disease and diabetes mellitus. I. The AGE concept". Cardiovascular Research. 37 (3): 586–600. doi: 10.1016/S0008-6363(97)00233-2. PMID 9659442.
- ^ Thornalley, P.J. (1996). "Advanced glycation and the development of diabetic complications. Unifying the involvement of glucose, methylglyoxal and oxidative stress". Endocrinol. Metab. 3: 149–166.
- ^ Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM (June 1999). "RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides". Cell. 97 (7): 889–901. doi: 10.1016/S0092-8674(00)80801-6. PMID 10399917. S2CID 7208198.
- ^ a b Ansari NA, Moinuddin, Ali R (2011). "Glycated lysine residues: a marker for non-enzymatic protein glycation in age-related diseases". Disease Markers. 30 (6): 317–324. doi: 10.1155/2011/718694. PMC 3825483. PMID 21725160.
- ^ a b Wells-Knecht KJ, Zyzak DV, Litchfield JE, Thorpe SR, Baynes JW (1995). "Mechanism of autoxidative glycosylation: identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose". Biochemistry. 34 (11): 3702–9. doi: 10.1021/bi00011a027. PMID 7893666.
- ^ Huttunen, Henri J.; Fages, Carole; Rauvala, Heikki (July 1999). "Receptor for Advanced Glycation End Products (RAGE)-mediated Neurite Outgrowth and Activation of NF-κB Require the Cytoplasmic Domain of the Receptor but Different Downstream Signaling Pathways". Journal of Biological Chemistry. 274 (28): 19919–19924. doi: 10.1074/jbc.274.28.19919.
- ^ Liu, Ting; Zhang, Lingyun; Joo, Donghyun; Sun, Shao-Cong (December 2017). "NF-κB signaling in inflammation". Signal Transduction and Targeted Therapy. 2 (1): 17023. doi: 10.1038/sigtrans.2017.23. PMC 5661633.
- ^ Ashraf, Jalaluddin Mohd.; Ahmad, Saheem; Choi, Inho; Ahmad, Nashrah; Farhan, Mohd.; Tatyana, Godovikova; Shahab, Uzma (November 2015). "Recent advances in detection of AGEs: Immunochemical, bioanalytical and biochemical approaches: Technological Progress in Age Detection". IUBMB Life. 67 (12): 897–913. doi: 10.1002/iub.1450.
- ^ a b c Gugliucci A, Mehlhaff K, Kinugasa E, et al. (2007). "Paraoxonase-1 concentrations in end-stage renal disease patients increase after hemodialysis: correlation with low molecular AGE adduct clearance". Clin. Chim. Acta. 377 (1–2): 213–20. doi: 10.1016/j.cca.2006.09.028. PMID 17118352.
- ^ Smedsrød B, Melkko J, Araki N, Sano H, Horiuchi S (1997). "Advanced glycation end products are eliminated by scavenger-receptor-mediated endocytosis in hepatic sinusoidal Kupffer and endothelial cells". Biochem. J. 322 (Pt 2): 567–73. doi: 10.1042/bj3220567. PMC 1218227. PMID 9065778.
- ^ Svistounov D, Smedsrød B (2004). "Hepatic clearance of advanced glycation end products (AGEs)—myth or truth?". J. Hepatol. 41 (6): 1038–40. doi: 10.1016/j.jhep.2004.10.004. PMID 15582139.
- ^ Koschinsky, Theodore; He, Ci-Jiang; Mitsuhashi, Tomoko; Bucala, Richard; Liu, Cecilia; Buenting, Christina; Heitmann, Kirsten; Vlassara, Helen (10 June 1997). "Orally absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy". Proceedings of the National Academy of Sciences of the United States of America. 94 (12): 6474–6479. Bibcode: 1997PNAS...94.6474K. doi: 10.1073/pnas.94.12.6474. PMC 21074. PMID 9177242.
- ^ a b Chaudhuri, Jyotiska; Bose, Neelanjan; Gong, Jianke; Hall, David; Rifkind, Alexander; Bhaumik, Dipa; Peiris, T. Harshani; Chamoli, Manish; Le, Catherine H.; Liu, Jianfeng; Lithgow, Gordon J.; Ramanathan, Arvind; Shawn Xu, X. Z.; Kapahi, Pankaj (21 November 2016). "A Caenorhabditis elegans Model Elucidates a Conserved Role for TRPA1-Nrf Signaling in Reactive Alpha-dicarbonyl Detoxification". Current Biology. 26 (22): 3014–3025. doi: 10.1016/j.cub.2016.09.024. PMC 5135008. PMID 27773573.
- ^ Mohmmad Abdul, Hafiz; Butterfield, D. Allan (February 2007). "Involvement of PI3K/PKG/ERK1/2 signaling pathways in cortical neurons to trigger protection by cotreatment of acetyl-L-carnitine and α-lipoic acid against HNE-mediated oxidative stress and neurotoxicity: Implications for Alzheimer's disease". Free Radical Biology and Medicine. 42 (3): 371–384. doi: 10.1016/j.freeradbiomed.2006.11.006. PMC 1808543. PMID 17210450.
- ^ Nandhini AT, Thirunavukkarasu V, Anuradha CV (August 2005). "Taurine prevents collagen abnormalities in high fructose-fed rats" (PDF). Indian J. Med. Res. 122 (2): 171–7. PMID 16177476. Archived from the original (PDF) on 2009-04-17. Retrieved 2009-04-16.
- ^ A. Gugliucci, " Sour Side of Sugar, A Glycation Web Page Archived July 1, 2007, at the Wayback Machine
- ^ Urios, P.; Grigorova-Borsos, A.-M.; Sternberg, M. (2007). "Aspirin inhibits the formation of... preview & related info". Diabetes Research and Clinical Practice. 77 (2): 337–340. doi: 10.1016/j.diabres.2006.12.024. PMID 17383766. Retrieved 2013-11-13.
- ^ Bucala, Richard; Cerami, Anthony (1992). Advanced Glycosylation: Chemistry, Biology, and Implications for Diabetes and Aging. Advances in Pharmacology. Vol. 23. pp. 1–34. doi: 10.1016/S1054-3589(08)60961-8. ISBN 978-0-12-032923-6. PMID 1540533.
- ^ Guiotto, Andrea; Calderan, Andrea; Ruzza, Paolo; Borin, Gianfranco (1 September 2005). "Carnosine and Carnosine-Related Antioxidants: A Review". Current Medicinal Chemistry. 12 (20): 2293–2315. doi: 10.2174/0929867054864796. PMID 16181134.
- ^ a b c Rahbar, S; Figarola, JL (2013-03-25). "Novel inhibitors of advanced glycation endproducts". Arch. Biochem. Biophys. 419 (1): 63–79. doi: 10.1016/j.abb.2003.08.009. PMID 14568010.
- ^ Mizutani, Kenichi; Ikeda, Katsumi; Yamori, Yukio (July 2000). "Resveratrol Inhibits AGEs-Induced Proliferation and Collagen Synthesis Activity in Vascular Smooth Muscle Cells from Stroke-Prone Spontaneously Hypertensive Rats". Biochemical and Biophysical Research Communications. 274 (1): 61–67. doi: 10.1006/bbrc.2000.3097. PMID 10903896.
- ^ Tang, Youcai; Chen, Anping (10 March 2014). "Curcumin eliminates the effect of advanced glycation end-products (AGEs) on the divergent regulation of gene expression of receptors of AGEs by interrupting leptin signaling". Laboratory Investigation. 94 (5): 503–516. doi: 10.1038/labinvest.2014.42. PMC 4006284. PMID 24614199.
- ^ Bakris, George L.; Bank, Alan J.; Kass, David A.; Neutel, Joel M.; Preston, Richard A.; Oparil, Suzanne (1 December 2004). "Advanced glycation end-product cross-link breakersA novel approach to cardiovascular pathologies related to the aging process". American Journal of Hypertension. 17 (S3): 23S–30S. doi: 10.1016/j.amjhyper.2004.08.022. PMID 15607432.
- ^ Vasan, Sara; Zhang, Xin; Zhang, Xini; Kapurniotu, Aphrodite; Bernhagen, Jürgen; Teichberg, Saul; Basgen, John; Wagle, Dilip; Shih, David; Terlecky, Ihor; Bucala, Richard; Cerami, Anthony; Egan, John; Ulrich, Peter (July 1996). "An agent cleaving glucose-derived protein crosslinks in vitro and in vivo". Nature. 382 (6588): 275–278. Bibcode: 1996Natur.382..275V. doi: 10.1038/382275a0. PMID 8717046. S2CID 4366953.
- ^ Jean, Daniel; Pouligon, Maryse; Dalle, Claude (2015). "Evaluation in vitro of AGE-crosslinks breaking ability of rosmarinic acid". Glycative Stress Research. 2 (4): 204–207. doi: 10.24659/gsr.2.4_204.
- ^ Monnier, Vincent M.; Mustata, Georgian T.; Biemel, Klaus L.; Reihl, Oliver; Lederer, Marcus O.; Zhenyu, Dai; Sell, David R. (June 2005). "Cross-Linking of the Extracellular Matrix by the Maillard Reaction in Aging and Diabetes: An Update on 'a Puzzle Nearing Resolution'". Annals of the New York Academy of Sciences. 1043 (1): 533–544. Bibcode: 2005NYASA1043..533M. doi: 10.1196/annals.1333.061. PMID 16037276. S2CID 27507321.
- ^ Furber, John D. (June 2006). "Extracellular Glycation Crosslinks: Prospects for Removal". Rejuvenation Research. 9 (2): 274–278. doi: 10.1089/rej.2006.9.274. PMID 16706655.