| Jadeite | |
|---|---|
 Jadeite from
Myanmar | |
| General | |
| Category | Pyroxene group |
|
Formula (repeating unit) | NaAlSi2O6 or Na(Al,Fe3+)Si2O6 |
| IMA symbol | Jd [1] |
| Strunz classification | 9.DA.25 |
| Crystal system | Monoclinic |
| Crystal class | Prismatic (2/m) (same H-M symbol) |
| Space group | C2/c |
| Identification | |
| Color | Apple-green, emerald-green, bluish green, leek-green, purplish blue, greenish white, white, black, may show green spots, rarely blue or violet; colorless in thin section. Often also banded. |
| Crystal habit | Commonly massive, or fibrous, granular; prismatic crystals rare |
| Twinning | Single and lamellar twinning on [100] and [001] |
| Cleavage | Good on [110] |
| Fracture | Splintery |
| Mohs scale hardness | 6.5–7 |
| Luster | Subvitreous, pearly on cleavages |
| Streak | White |
| Diaphaneity | Translucent |
| Specific gravity | 3.24 to 3.43 |
| Polish luster | vitreous to greasy [2] |
| Optical properties | Biaxial (+) |
| Refractive index | nα = 1.654 – 1.673 nβ = 1.659 – 1.679 nγ = 1.667 – 1.693 |
| Birefringence | δ = 0.013 – 0.020 |
| Dispersion | r > v; moderate to strong. |
| Ultraviolet fluorescence | Dark colors are generally inert. Light green – inert to weak white in long wave, generally inert in short wave; light yellow – inert to weak green in long wave, generally inert in short wave; white – inert to weak in long wave, generally inert in short wave; light purple – inert to weak white or weak brownish red in long wave, generally inert in short wave; some dyed lavender colors – moderate to strong orange in long wave, weaker in short wave [2] |
| References | [3] [4] [5] |
Jadeite is a pyroxene mineral with composition Na Al Si2 O6. It is hard ( Mohs hardness of about 6.5 to 7.0), very tough, and dense, with a specific gravity of about 3.4. It is found in a wide range of colors, but is most often found in shades of green or white. Jadeite is formed only in the subduction zones of continental margins, where rock undergoes metamorphism at high pressure but relatively low temperature.
Jadeite is the principal mineral making up the most valuable form of jade, a precious stone particularly prized in China. Most gem-quality jadeite jade comes from northern Myanmar. Jade tools and implements have been found at Stone Age sites, showing that the mineral has been prized by humans since before the beginning of written history.
Name
The name jadeite is derived (via French: jade and Latin: ilia [6]) from the Spanish phrase "piedra de ijada" which means "stone of the side". The Latin version of the name, lapis nephriticus, is the origin of the term nephrite, which is a different mineral that also shares the common name jade. [7]
Properties
Jadeite is a hard, extremely tough, [8] rare mineral of the clinopyroxene family of minerals. [9] Though highly variable in color, [10] it is typically apple-green to emerald-green, or less commonly white or white with spots of green. [9] Occurrences are typically granular or massive; [9] individual crystals are very rare, occurring only as small prismatic crystals in vugs in massive jadeite. [8] Crystals are four-sided or eight-sided in cross section and show perfect cleavage on [110] at angles of 87 and 93 degrees. There is also a rare parting on [100]. The interlocking crystals of massive jadeite help give it its extreme toughness. [11]
Jadeite has a Mohs hardness of 6.5 to 7, slightly less than that of common quartz. [9] Fracture surfaces are sugary in texture, and sparkle from the exposed perfect cleavage on [110]. Jade is relatively dense, with a specific gravity of 3.3 to 3.5 in natural specimens. [9] The specific gravity increases with the iron content, and very pure jadeite has a specific gravity of 3.25. [8] The luster is vitreous, or pearly on exposed cleavage surfaces, and the streak is colorless. [9] Jadeite is translucent to transparent. [8]
Jadeite is characterized by its green color and tough aggregates of compact fibrous crystals. It can be distinguished from nephrite by its vitreous luster on polished surfaces (polished nephrite has an oily luster) [9] and by its higher density and refractive index. Serpentine also has a lower density and refractive index than jadeite. Massive jadeite also characteristically shows a more granular texture than nephrite or serpentinite. [8]
Jadeite has a fusibility of 2.5 (making it moderately easy to fuse with a propane flame) and gives a yellow flame color. [8]
Pure jadeite has the composition NaAlSi2O6 and has the typical clinopyroxene structure. This consists of long chains of silica tetrahedra in which each silicon ion is surrounded by four oxygen ions, with two of the oxygen ions shared with neighboring silica tetraheda. The chains are bonded together by aluminium and sodium ions to form the full three-dimensional structure of a jadeite crystal. The aluminium joins pairs of chains (occupying the so-called M1 site) while sodium joins the paired chains to neighboring paired chains (occupying the so-called M2 site). The resulting crystal structure belongs to the monoclinic system, [9] with space group C2/c. [3]
Chemistry and origin
Pure jadeite has the composition NaAlSi2O6. There is no significant replacement of silicon by aluminium in natural jadeite, and only very limited substitution of ferric iron for aluminium. [9] However, calcium substitutes for up to 20% of the sodium, balanced by substitution of magnesium or ferrous iron for aluminium. [11]
Omphacite is intermediate in composition between jadeite and diopside. [9] However, there is not a true solid solution series, as omphacite has its own structure that is slightly different from either pure jadeite or pure diopside, so that it is separated from either end member by a miscibility gap. [11] Chloromelanite is a dark green variety of jadeite in which some aluminium is replaced by iron, while imperial jade, the most valuable variety of jade, is colored an intense emerald green by traces of chromium. [8]
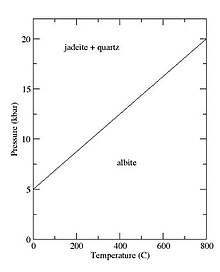
Jadeite occurs with albite in metamorphic rock of the low-temperature, high-pressure blueschist facies at destructive plate margins. [9] Although it is intermediate in silica content between albite and nepheline, it is not stable under the conditions in which these two minerals are present. Formation of jadeite requires a pressure of 10 to 25 kbar and a temperature of 600 to 1,000 °C (1,100 to 1,800 °F) via the reaction: [9]
-
+ →(Reaction 1)
Jadeite can also form at high pressure via the reaction: [9]
-
→ +(Reaction 2)
At still higher pressure, corresponding to the highest blueschist facies, jadeite reacts with lawsonite to form zoisite and paragonite: [12]
-
+ → + +(Reaction 3)
Minerals associated with jadeite include: glaucophane, lawsonite, muscovite, aragonite, serpentine and quartz. [9]
Rocks that consist almost entirely of jadeite are called jadeitite. In all well-documented occurrences, jadeitite appears to have formed from subduction zone fluids in association with serpentinite. [13]
Jadeitite is resistant to weathering, and alluvial boulders of jadeitite released from the serpentine-rich environments in which they formed can have weights of up to tons. Raw specimens having Burmese tax stamps or polished slots for evaluating quality are prized by some collectors. [8]
Colors


Jadeite's color commonly ranges from white through pale apple green to deep jade green but can also be blue-green (like the recently rediscovered "Olmec Blue" jade), pink, lavender and a multitude of other rare colors. Chloromelanite is a very dark green to black variety. [14] Color is largely affected by the presence of trace elements such as chromium and iron. Its translucence varies from opaque to almost clear. [8] Variations in color and translucence are often found even within a single specimen. [9]
Occurrence
Significant occurrences of jadeite are isolated and rare. [15] It is found exclusively in high-pressure, low-temperature metamorphic rock of continental margins. [9] Here it may be found as pods or veins or as disseminated grains. [11] Deposits are found in Myanmar, the Alps, Russia, [16] California, [9] Japan, [17] and Guatemala. [8] In the Franciscan Complex of California, jadeite is associated with glaucophane, aragonite, muscovite, lawsonite, and quartz. [9] However, occurrences of lapidary quality are almost exclusive to Myanmar. [8] Stream boulders of the Uyu River remain an important source of jadeite. [18]
Uses
Jadeite is the dominant mineral of the most desirable variety of jade. [10] This was prized in traditional Chinese culture, where it was worked into a great variety of beautiful ornaments and utensils. [9]
Jadeite was also used by Stone Age peoples for implements and weapons. [9]
Jade received its name, "piedra de ijada" ("stone of the side"), because it was once thought to cure kidney ailments when applied to the side of the body. [8]
Jade
Jadeite is one of two minerals recognized as the gemstone jade. The other is nephrite. [19] Occasionally, other minerals such as serpentine or quartz are sold as jade, but the difference can be determined by cleavage and hardness. [20] Jadeite jade is the most valuable form, [8] with the highest-quality material commanding prices well in excess of $200 per carat as of 1994 [update]. [21] Jadeite jade first came into significant use in China only towards the end of the 18th century, as fei tsui. [22]
Jadeite from the Motagua Valley, Guatemala, was used by the Olmec and Maya peoples, as well as the indigenous peoples of Costa Rica. [23]
Unusual colors, like "Olmec blue" jade, which is characterized by its deep blue-green, translucent hue with white flecking, are becoming more highly valued because of its unique beauty and historical use by the Mesoamerican Olmec and also in Costa Rica. [24]
Stone Age use

Over 180 axe heads made from jadeitite quarried in northern Italy in the Neolithic era have been found across the British Isles. [25] [26] Because of the difficulty of working this material, all the axe heads of this type found are thought to have been non-utilitarian and to have represented some form of currency or be the products of gift exchange. [27]
A great many jadeite beads and axe heads as well as the remains of jadeite workshops from the Neolithic era have been uncovered in Itoigawa, Japan. These beads and axes were traded throughout Japan and the Korean Peninsula and were produced by the world's oldest known jadeite-using culture, centered on the Itoigawa region. [28] [29]

See also
References
- ^ Warr, L.N. (2021). "IMA–CNMNC approved mineral symbols". Mineralogical Magazine. 85 (3): 291–320. Bibcode: 2021MinM...85..291W. doi: 10.1180/mgm.2021.43. S2CID 235729616.
- ^ a b (Gia), Gemological. Gem Reference Guide. City: Gemological Institute of America (GIA), 1988. ISBN 0-87311-019-6
- ^ a b "Handbook of Mineralogy : Jadeite Na(Al; Fe3+)Si2O6" (PDF). Rruff.geo.arizona.edu. Retrieved 25 January 2022.
- ^ "Jadeite: Mineral information, data and localities". Mindat.org.
- ^ "A to Z List". Webmineral.com.
- ^ "Jade | Etymology, origin and meaning of jade by etymonline". Etymonline.com.
- ^ Delario, A. J. (November 1960). "Jade through the Ages — Part 1". Rocks & Minerals. 35 (11–12): 578–582. Bibcode: 1960RoMin..35..578D. doi: 10.1080/00357529.1960.11766693.
- ^ a b c d e f g h i j k l m Sinkankas, John (1964). Mineralogy for amateurs. Princeton, N.J.: Van Nostrand. ISBN 0442276249.
- ^ a b c d e f g h i j k l m n o p q r s t Klein, Cornelis; Hurlbut, Cornelius S. Jr. (1993). Manual of mineralogy : (after James D. Dana) (21st ed.). New York: Wiley. pp. 482–483, 598. ISBN 047157452X.
- ^ a b Jackson, Julia A., ed. (1997). "jadeite". Glossary of geology (Fourth ed.). Alexandria, Virginia: American Geological Institute. ISBN 0922152349.
- ^ a b c d Nesse, William D. (2000). Introduction to mineralogy. New York: Oxford University Press. pp. 271=272. ISBN 9780195106916.
- ^ Yardley 1989, p. 105.
- ^ Sorena Sorensen, George E. Harlow, and Douglas Rumble, The origin of jadeitite-forming subduction-zone fluids: CL-guided SIMS oxygen-isotope and trace-element evidence. American Mineralogist, v. 91, pp. 979-996 (2006)
- ^ "Chloromelanite". Mindat.org. Retrieved 25 January 2022.
- ^ Allaby, Michael (2013). A dictionary of geology and earth sciences (Fourth ed.). Oxford: Oxford University Press. ISBN 9780199653065.
- ^ Yingying, Xing; Lijian, Qi (31 March 2021). "Structural hydroxyl distribution in jadeite grains and the diagenesis mechanism of jadeitite in Myanmar, Guatemala and Russia". Materials Testing. 63 (3): 235–244. Bibcode: 2021MTest..63..235Y. doi: 10.1515/mt-2020-0034. ISSN 2195-8572. S2CID 232433079.
- ^ "Itoigawa City, Niigata Prefecture, Chubu Region, Honshu Island, Japan". Mindat.org. Retrieved 2016-06-30.
- ^ Klein & Hurlbut 1993, p. 599.
- ^ Klein & Hurlbut 1993, pp. 598–599.
- ^ Desautels, Paul E. (1986). "False Jade". The Jade Kingdom. pp. 9–11. doi: 10.1007/978-1-4684-6572-3_2. ISBN 978-1-4684-6574-7.
- ^ Austin, G.T. (1994). Gemstones (PDF). US Department of the Interior, Bureau of Mines.
- ^ Keverne, Roger, ed. (1991). Jade. New York: Springer Science+Business Media. p. 54. ISBN 9781461539223.
- ^ Hargett, D. (1990). "Jadeite of Guatemala: A contemporary view" (PDF). Gems Gemol. 26 (2): 134–141. doi: 10.5741/GEMS.26.2.134. Retrieved 24 December 2021.
- ^ Easby, Elizabeth Kennedy. Pre-Columbian Jade from Costa Rica. (1968). André Emmerich Inc., New York
- ^ "Jadeite axe-head". British Museum. Retrieved 21 November 2009.
- ^ "Jadeite axe". Wiltshire Heritage Museum. Archived from the original on 25 April 2012. Retrieved 31 October 2011.
- ^ Barker, Graeme (1999). Companion encyclopedia of archaeology. New York: Routledge. pp. 378. ISBN 0-415-21329-0.
- ^ Kijima, Tsutomu. "翡翠製大珠の加工と流通". Tateshinakougen.gr.jp (in Japanese).
- ^ Pearson, Richard. "International Jomon Culture Conference Bulletin 1 2004 (English version)". Academia.edu. Retrieved 2016-06-30.
Sources
- Yardley, Bruce W. D. (1989). An Introduction to Metamorphic Petrology. Longman Earth Science Series. Harlow, Essex, England: Longman Scientific & Technical. ISBN 0-582-30096-7.
External links
-
 Media related to
Jadeite at Wikimedia Commons
Media related to
Jadeite at Wikimedia Commons